Gsy, a novel glucansucrase from Leuconostoc mesenteroides, mediates the formation of cell aggregates in response to oxidative stress
- PMID: 27924943
- PMCID: PMC5141493
- DOI: 10.1038/srep38122
Gsy, a novel glucansucrase from Leuconostoc mesenteroides, mediates the formation of cell aggregates in response to oxidative stress
Abstract
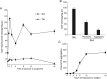
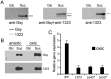
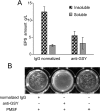
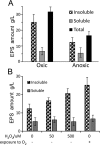
Leuconostoc mesenteroides is a member of lactic acid bacteria (LAB) with wide applications in the food and medical industries. Species in the genus Leuconostoc are catalase-negative and generally regarded as facultative anaerobic or aerotolerant organisms. Despite their extensive use in industry, certain issues concerning the aerobic life of L. mesenteroides, e.g., the mechanism involved in the tolerance to oxygen, remain to be addressed. In this manuscript, a survival strategy employed by L. mesenteroides BD3749 in response to oxidative stress was elucidated. BD3749 cells cultivated in medium with sucrose available synthesized large amounts of exopolysaccharides, mostly consisting of insoluble EPS. When BD3749 cells were challenged with oxidative stress, the amount of insoluble EPS was greatly enhanced. The synthesized EPSs reduced the accumulation of reactive oxygen species (ROS) in bacterial cells and improved their survival during chronic oxidative stress. Another study showed that Gsy, a novel glucansucrase in the GH70 family that is induced by sucrose and up-regulated following exposure to oxygen, was responsible for the synthesis of insoluble EPS. Gsy was subsequently demonstrated to play pivotal roles in the formation of aggregates to alleviate the detrimental effects on BD3749 cells exerted by oxygen.
Figures



Similar articles
-
Molecular and Functional Study of a Branching Sucrase-Like Glucansucrase Reveals an Evolutionary Intermediate between Two Subfamilies of the GH70 Enzymes.Appl Environ Microbiol. 2018 Apr 16;84(9):e02810-17. doi: 10.1128/AEM.02810-17. Print 2018 May 1. Appl Environ Microbiol. 2018. PMID: 29453261 Free PMC article.
-
Extrusion of Dissolved Oxygen by Exopolysaccharide From Leuconostoc mesenteroides and Its Implications in Relief of the Oxygen Stress.Front Microbiol. 2018 Oct 17;9:2467. doi: 10.3389/fmicb.2018.02467. eCollection 2018. Front Microbiol. 2018. PMID: 30405549 Free PMC article.
-
Optimization and purification of glucansucrase produced by Leuconostoc mesenteroides DRP2-19 isolated from Chinese Sauerkraut.Prep Biochem Biotechnol. 2018;48(6):465-473. doi: 10.1080/10826068.2018.1466149. Epub 2018 Jun 11. Prep Biochem Biotechnol. 2018. PMID: 29889600
-
Extracellular polysaccharides produced by bacteria of the Leuconostoc genus.World J Microbiol Biotechnol. 2020 Sep 29;36(11):161. doi: 10.1007/s11274-020-02937-9. World J Microbiol Biotechnol. 2020. PMID: 32989599 Review.
-
Microbial oxidative stress response: Novel insights from environmental facultative anaerobic bacteria.Arch Biochem Biophys. 2015 Oct 15;584:28-35. doi: 10.1016/j.abb.2015.08.012. Epub 2015 Aug 28. Arch Biochem Biophys. 2015. PMID: 26319291 Review.
Cited by
-
Glycosyltransferases Expression Changes in Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 Grown on Different Carbon Sources.Foods. 2023 May 4;12(9):1893. doi: 10.3390/foods12091893. Foods. 2023. PMID: 37174431 Free PMC article.
-
Different Modes of Regulation of the Expression of Dextransucrase in Leuconostoc lactis AV1n and Lactobacillus sakei MN1.Front Microbiol. 2019 May 7;10:959. doi: 10.3389/fmicb.2019.00959. eCollection 2019. Front Microbiol. 2019. PMID: 31134012 Free PMC article.
-
Molecular and Functional Study of a Branching Sucrase-Like Glucansucrase Reveals an Evolutionary Intermediate between Two Subfamilies of the GH70 Enzymes.Appl Environ Microbiol. 2018 Apr 16;84(9):e02810-17. doi: 10.1128/AEM.02810-17. Print 2018 May 1. Appl Environ Microbiol. 2018. PMID: 29453261 Free PMC article.
-
Response of Controlled Cell Load Biofilms to Cold Atmospheric Plasma Jet: Evidence of Extracellular Matrix Contribution.Life (Basel). 2021 Jul 15;11(7):694. doi: 10.3390/life11070694. Life (Basel). 2021. PMID: 34357067 Free PMC article.
-
Extrusion of Dissolved Oxygen by Exopolysaccharide From Leuconostoc mesenteroides and Its Implications in Relief of the Oxygen Stress.Front Microbiol. 2018 Oct 17;9:2467. doi: 10.3389/fmicb.2018.02467. eCollection 2018. Front Microbiol. 2018. PMID: 30405549 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

