Biliary Secretion of Quasi-Enveloped Human Hepatitis A Virus
- PMID: 27923925
- PMCID: PMC5142623
- DOI: 10.1128/mBio.01998-16
Biliary Secretion of Quasi-Enveloped Human Hepatitis A Virus
Abstract
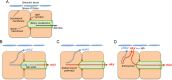
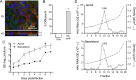
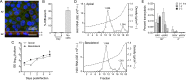
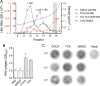
Hepatitis A virus (HAV) is an unusual picornavirus that is released from cells cloaked in host-derived membranes. These quasi-enveloped virions (eHAV) are the only particle type circulating in blood during infection, whereas only nonenveloped virions are shed in feces. The reason for this is uncertain. Hepatocytes, the only cell type known to support HAV replication in vivo, are highly polarized epithelial cells with basolateral membranes facing onto hepatic (blood) sinusoids and apical membranes abutting biliary canaliculi from which bile is secreted to the gut. To assess whether eHAV and nonenveloped virus egress from cells via vectorially distinct pathways, we studied infected polarized cultures of Caco-2 and HepG2-N6 cells. Most (>99%) progeny virions were released apically from Caco-2 cells, whereas basolateral (64%) versus apical (36%) release was more balanced with HepG2-N6 cells. Both apically and basolaterally released virions were predominantly enveloped, with no suggestion of differential vectorial release of eHAV versus naked virions. Basolateral to apical transcytosis of either particle type was minimal (<0.02%/h) in HepG2-N6 cells, arguing against this as a mechanism for differences in membrane envelopment of serum versus fecal virus. High concentrations of human bile acids converted eHAV to nonenveloped virions, whereas virus present in bile from HAV-infected Ifnar1-/- Ifngr1-/- and Mavs-/- mice banded over a range of densities extending from that of eHAV to that of nonenveloped virions. We conclude that nonenveloped virions shed in feces are derived from eHAV released across the canalicular membrane and stripped of membranes by the detergent action of bile acids within the proximal biliary canaliculus.
Importance: HAV is a hepatotropic, fecally/orally transmitted picornavirus that can cause severe hepatitis in humans. Recent work reveals that it has an unusual life cycle. Virus is found in cell culture supernatant fluids in two mature, infectious forms: one wrapped in membranes (quasi-enveloped) and another that is nonenveloped. Membrane-wrapped virions circulate in blood during acute infection and are resistant to neutralizing antibodies, likely facilitating HAV dissemination within the liver. On the other hand, virus shed in feces is nonenveloped and highly stable, facilitating epidemic spread and transmission to naive hosts. Factors controlling the biogenesis of these two distinct forms of the virus in infected humans are not understood. Here we characterize vectorial release of quasi-enveloped virions from polarized epithelial cell cultures and provide evidence that bile acids strip membranes from eHAV following its secretion into the biliary tract. These results enhance our understanding of the life cycle of this unusual picornavirus.
Copyright © 2016 Hirai-Yuki et al.
Figures
Similar articles
-
TIM1 (HAVCR1) Is Not Essential for Cellular Entry of Either Quasi-enveloped or Naked Hepatitis A Virions.mBio. 2017 Sep 5;8(5):e00969-17. doi: 10.1128/mBio.00969-17. mBio. 2017. PMID: 28874468 Free PMC article.
-
Vectorial Release of Hepatitis E Virus in Polarized Human Hepatocytes.J Virol. 2019 Feb 5;93(4):e01207-18. doi: 10.1128/JVI.01207-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463960 Free PMC article.
-
Cellular entry and uncoating of naked and quasi-enveloped human hepatoviruses.Elife. 2019 Feb 25;8:e43983. doi: 10.7554/eLife.43983. Elife. 2019. PMID: 30801249 Free PMC article.
-
Quasi-enveloped hepatitis virus assembly and release.Adv Virus Res. 2020;108:315-336. doi: 10.1016/bs.aivir.2020.08.004. Epub 2020 Sep 28. Adv Virus Res. 2020. PMID: 33837720 Review.
-
Cell entry and release of quasi-enveloped human hepatitis viruses.Nat Rev Microbiol. 2023 Sep;21(9):573-589. doi: 10.1038/s41579-023-00889-z. Epub 2023 Apr 25. Nat Rev Microbiol. 2023. PMID: 37185947 Free PMC article. Review.
Cited by
-
A Useful Method to Provide Infectious and Cultivable In Vitro Naked Viral Particles of Hepatitis A Virus.Viruses. 2024 Aug 26;16(9):1360. doi: 10.3390/v16091360. Viruses. 2024. PMID: 39339837 Free PMC article.
-
Biochemical analysis of the host factor activity of ZCCHC14 in hepatitis A virus replication.J Virol. 2024 Apr 16;98(4):e0005724. doi: 10.1128/jvi.00057-24. Epub 2024 Mar 19. J Virol. 2024. PMID: 38501662 Free PMC article.
-
Loxapine inhibits replication of hepatitis A virus in vitro and in vivo by targeting viral protein 2C.PLoS Pathog. 2024 Mar 13;20(3):e1012091. doi: 10.1371/journal.ppat.1012091. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38478584 Free PMC article.
-
Hepatoviruses promote very-long-chain fatty acid and sphingolipid synthesis for viral RNA replication and quasi-enveloped virus release.Sci Adv. 2023 Oct 20;9(42):eadj4198. doi: 10.1126/sciadv.adj4198. Epub 2023 Oct 20. Sci Adv. 2023. PMID: 37862421 Free PMC article.
-
Hepatitis A virus infection.Nat Rev Dis Primers. 2023 Sep 28;9(1):51. doi: 10.1038/s41572-023-00461-2. Nat Rev Dis Primers. 2023. PMID: 37770459 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
