Cap analogs modified with 1,2-dithiodiphosphate moiety protect mRNA from decapping and enhance its translational potential
- PMID: 27903882
- PMCID: PMC5175369
- DOI: 10.1093/nar/gkw896
Cap analogs modified with 1,2-dithiodiphosphate moiety protect mRNA from decapping and enhance its translational potential
Abstract
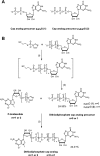
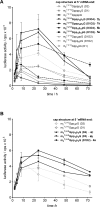
Along with a growing interest in mRNA-based gene therapies, efforts are increasingly focused on reaching the full translational potential of mRNA, as a major obstacle for in vivo applications is sufficient expression of exogenously delivered mRNA. One method to overcome this limitation is chemically modifying the 7-methylguanosine cap at the 5' end of mRNA (m7Gppp-RNA). We report a novel class of cap analogs designed as reagents for mRNA modification. The analogs carry a 1,2-dithiodiphosphate moiety at various positions along a tri- or tetraphosphate bridge, and thus are termed 2S analogs. These 2S analogs have high affinities for translation initiation factor 4E, and some exhibit remarkable resistance against the SpDcp1/2 decapping complex when introduced into RNA. mRNAs capped with 2S analogs combining these two features exhibit high translation efficiency in cultured human immature dendritic cells. These properties demonstrate that 2S analogs are potentially beneficial for mRNA-based therapies such as anti-cancer immunization.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

Similar articles
-
Synthesis and properties of mRNA cap analogs containing imidodiphosphate moiety--fairly mimicking natural cap structure, yet resistant to enzymatic hydrolysis.Bioorg Med Chem. 2012 Mar 1;20(5):1699-710. doi: 10.1016/j.bmc.2012.01.013. Epub 2012 Jan 20. Bioorg Med Chem. 2012. PMID: 22316555
-
mRNA cap analogues substituted in the tetraphosphate chain with CX2: identification of O-to-CCl2 as the first bridging modification that confers resistance to decapping without impairing translation.Nucleic Acids Res. 2017 Sep 6;45(15):8661-8675. doi: 10.1093/nar/gkx569. Nucleic Acids Res. 2017. PMID: 28666355 Free PMC article.
-
Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability.Methods Enzymol. 2007;431:203-27. doi: 10.1016/S0076-6879(07)31011-2. Methods Enzymol. 2007. PMID: 17923237 Review.
-
5'-Phosphorothiolate Dinucleotide Cap Analogues: Reagents for Messenger RNA Modification and Potent Small-Molecular Inhibitors of Decapping Enzymes.J Am Chem Soc. 2018 May 9;140(18):5987-5999. doi: 10.1021/jacs.8b02597. Epub 2018 May 1. J Am Chem Soc. 2018. PMID: 29676910
-
Synthetic mRNAs with superior translation and stability properties.Methods Mol Biol. 2013;969:55-72. doi: 10.1007/978-1-62703-260-5_4. Methods Mol Biol. 2013. PMID: 23296927 Review.
Cited by
-
mRNA - A game changer in regenerative medicine, cell-based therapy and reprogramming strategies.Adv Drug Deliv Rev. 2021 Dec;179:114002. doi: 10.1016/j.addr.2021.114002. Epub 2021 Oct 13. Adv Drug Deliv Rev. 2021. PMID: 34653534 Free PMC article. Review.
-
Chemically modified mRNA beyond COVID-19: Potential preventive and therapeutic applications for targeting chronic diseases.Biomed Pharmacother. 2022 Jan;145:112385. doi: 10.1016/j.biopha.2021.112385. Epub 2021 Oct 28. Biomed Pharmacother. 2022. PMID: 34915673 Free PMC article. Review.
-
Trinucleotide cap analogs with triphosphate chain modifications: synthesis, properties, and evaluation as mRNA capping reagents.Nucleic Acids Res. 2024 Oct 14;52(18):10788-10809. doi: 10.1093/nar/gkae763. Nucleic Acids Res. 2024. PMID: 39248095 Free PMC article.
-
Nurturing Deep Tech to Solve Social Problems: Learning from COVID-19 mRNA Vaccine Development.Pathogens. 2022 Dec 5;11(12):1469. doi: 10.3390/pathogens11121469. Pathogens. 2022. PMID: 36558803 Free PMC article. Review.
-
An RNA toolbox for cancer immunotherapy.Nat Rev Drug Discov. 2018 Oct;17(10):751-767. doi: 10.1038/nrd.2018.132. Epub 2018 Sep 7. Nat Rev Drug Discov. 2018. PMID: 30190565 Review.
References
-
- Quabius E., Krupp G. Synthetic mRNAs for manipulating cellular phenotypes: an overview. N. Biotechnol. 2014;32:229–235. - PubMed
-
- Deering R., Kommareddy S. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert Opin. Drug Deliv. 2014;11:885–899. - PubMed
-
- Saunders A., Wang J. Export and expression: mRNAs deliver new messages for controlling pluripotency. Cell Stem Cell. 2014;14:549–550. - PubMed
-
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
-
- Astakhova I.K., Wengel J. Scaffolding along nucleic acid duplexes using 2′-amino-locked nucleic acids. Acc. Chem. Res. 2014;47:1768–1777. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

