Postreplication Roles of the Brucella VirB Type IV Secretion System Uncovered via Conditional Expression of the VirB11 ATPase
- PMID: 27899503
- PMCID: PMC5137499
- DOI: 10.1128/mBio.01730-16
Postreplication Roles of the Brucella VirB Type IV Secretion System Uncovered via Conditional Expression of the VirB11 ATPase
Abstract
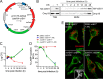
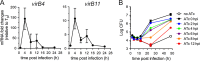
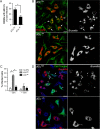
Brucella abortus, the bacterial agent of the worldwide zoonosis brucellosis, primarily infects host phagocytes, where it undergoes an intracellular cycle within a dedicated membrane-bound vacuole, the Brucella-containing vacuole (BCV). Initially of endosomal origin (eBCV), BCVs are remodeled into replication-permissive organelles (rBCV) derived from the host endoplasmic reticulum, a process that requires modulation of host secretory functions via delivery of effector proteins by the Brucella VirB type IV secretion system (T4SS). Following replication, rBCVs are converted into autophagic vacuoles (aBCVs) that facilitate bacterial egress and subsequent infections, arguing that the bacterium sequentially manipulates multiple cellular pathways to complete its cycle. The VirB T4SS is essential for rBCV biogenesis, as VirB-deficient mutants are stalled in eBCVs and cannot mediate rBCV biogenesis. This has precluded analysis of whether the VirB apparatus also drives subsequent stages of the Brucella intracellular cycle. To address this issue, we have generated a B. abortus strain in which VirB T4SS function is conditionally controlled via anhydrotetracycline (ATc)-dependent complementation of a deletion of the virB11 gene encoding the VirB11 ATPase. We show in murine bone marrow-derived macrophages (BMMs) that early VirB production is essential for optimal rBCV biogenesis and bacterial replication. Transient expression of virB11 prior to infection was sufficient to mediate normal rBCV biogenesis and bacterial replication but led to T4SS inactivation and decreased aBCV formation and bacterial release, indicating that these postreplication stages are also T4SS dependent. Hence, our findings support the hypothesis of additional, postreplication roles of type IV secretion in the Brucella intracellular cycle.
Importance: Many intracellular bacterial pathogens encode specialized secretion systems that deliver effector proteins into host cells to mediate the multiple stages of their intracellular cycles. Because these intracellular events occur sequentially, classical genetic approaches cannot address the late roles that these apparatuses play, as secretion-deficient mutants cannot proceed past their initial defect. Here we have designed a functionally controllable VirB type IV secretion system (T4SS) in the bacterial pathogen Brucella abortus to decipher its temporal requirements during the bacterium's intracellular cycle in macrophages. By controlling production of the VirB11 ATPase, which energizes the T4SS, we show not only that this apparatus is required early to generate the Brucella replicative organelle but also that it contributes to completion of the bacterium's cycle and bacterial egress. Our findings expand upon the pathogenic functions of the Brucella VirB T4SS and illustrate targeting of secretion ATPases as a useful strategy to manipulate the activity of bacterial secretion systems.
Copyright © 2016 Smith et al.
Figures


Similar articles
-
A T4SS Effector Targets Host Cell Alpha-Enolase Contributing to Brucella abortus Intracellular Lifestyle.Front Cell Infect Microbiol. 2016 Nov 16;6:153. doi: 10.3389/fcimb.2016.00153. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27900285 Free PMC article.
-
Epistatic Interplay between Type IV Secretion Effectors Engages the Small GTPase Rab2 in the Brucella Intracellular Cycle.mBio. 2020 Mar 31;11(2):e03350-19. doi: 10.1128/mBio.03350-19. mBio. 2020. PMID: 32234817 Free PMC article.
-
A Brucella Type IV Effector Targets the COG Tethering Complex to Remodel Host Secretory Traffic and Promote Intracellular Replication.Cell Host Microbe. 2017 Sep 13;22(3):317-329.e7. doi: 10.1016/j.chom.2017.07.017. Epub 2017 Aug 24. Cell Host Microbe. 2017. PMID: 28844886 Free PMC article.
-
The VirB System Plays a Crucial Role in Brucella Intracellular Infection.Int J Mol Sci. 2021 Dec 20;22(24):13637. doi: 10.3390/ijms222413637. Int J Mol Sci. 2021. PMID: 34948430 Free PMC article. Review.
-
Type IV secretion system of Brucella spp. and its effectors.Front Cell Infect Microbiol. 2015 Oct 13;5:72. doi: 10.3389/fcimb.2015.00072. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26528442 Free PMC article. Review.
Cited by
-
Characterization of a Novel Diarrheagenic Strain of Proteus mirabilis Associated With Food Poisoning in China.Front Microbiol. 2019 Dec 12;10:2810. doi: 10.3389/fmicb.2019.02810. eCollection 2019. Front Microbiol. 2019. PMID: 31921012 Free PMC article.
-
Subversion of the Endocytic and Secretory Pathways by Bacterial Effector Proteins.Front Cell Dev Biol. 2018 Jan 24;6:1. doi: 10.3389/fcell.2018.00001. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 29417046 Free PMC article. Review.
-
Harnessing Macrophages for Controlled-Release Drug Delivery: Lessons From Microbes.Front Pharmacol. 2019 Jan 25;10:22. doi: 10.3389/fphar.2019.00022. eCollection 2019. Front Pharmacol. 2019. PMID: 30740053 Free PMC article. Review.
-
The Intracellular Life Cycle of Brucella spp.Microbiol Spectr. 2019 Mar;7(2):10.1128/microbiolspec.bai-0006-2019. doi: 10.1128/microbiolspec.BAI-0006-2019. Microbiol Spectr. 2019. PMID: 30848234 Free PMC article. Review.
-
Controlled Activity of the Salmonella Invasion-Associated Injectisome Reveals Its Intracellular Role in the Cytosolic Population.mBio. 2017 Dec 5;8(6):e01931-17. doi: 10.1128/mBio.01931-17. mBio. 2017. PMID: 29208746 Free PMC article.
References
-
- Green ER, Mecsas J. 2016. Bacterial secretion systems: an overview, p 215–239. In Kudva I, Cornick N, Plummer P, Zhang Q, Nicholson T, Bannantine J, Bellaire B (ed), Virulence mechanisms of bacterial pathogens. ASM Press, Washington, DC. doi:10.1128/microbiolspec.VMBF-0012-2015. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
