Angiotensin-(1-7) protects cardiomyocytes against high glucose-induced injuries through inhibiting reactive oxygen species-activated leptin-p38 mitogen-activated protein kinase/extracellular signal-regulated protein kinase 1/2 pathways, but not the leptin-c-Jun N-terminal kinase pathway in vitro
- PMID: 27896943
- PMCID: PMC5497033
- DOI: 10.1111/jdi.12603
Angiotensin-(1-7) protects cardiomyocytes against high glucose-induced injuries through inhibiting reactive oxygen species-activated leptin-p38 mitogen-activated protein kinase/extracellular signal-regulated protein kinase 1/2 pathways, but not the leptin-c-Jun N-terminal kinase pathway in vitro
Abstract
Aims/introduction: Angiotensin-(1-7) (Ang-[1-7]), recognized as a new bioactive peptide in the renin-angiotensin system, shows biological and pharmacological properties in diabetic cardiovascular diseases. The leptin-induced p38 mitogen-activated protein kinase (MAPK) pathway has been reported to contribute to high glucose (HG)-induced injury. In the present study, we showed the mechanism of how Ang-(1-7) can protect against HG-stimulated injuries in H9c2 cells.
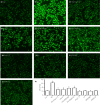
Materials and methods: H9c2 cells were treated with 35 mmol/L glucose (HG) for 24 h to establish a model of HG-induced damage. Apoptotic cells were observed by Hoechst 33258 staining. Cell viability was analyzed by cell counter kit-8. The expression of protein was detected by western blot. Reactive oxygen species was tested by 2',7'-dichlorodihydrofluorescein diacetate staining. Mitochondrial membrane potential was measured by 5,5',6,6'-Tetrachloro-1,1',3,3'-tetraethyl-imidacarbocyanine iodide staining.
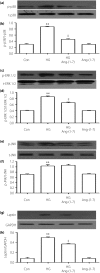
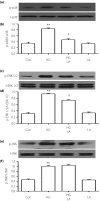
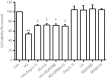
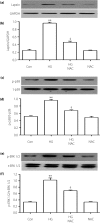
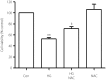
Results: The present results showed that treating H9c2 cells with HG obviously enhanced the expressions of both the leptin and phosphorylated (p)-MAPK pathway. However, the overexpression levels of leptin and p-p38 MAPK/p-extracellular signal-regulated protein kinase 1/2 (ERK1/2), but not p-c-Jun N-terminal kinase, were significantly suppressed by treatment of the cells with Ang-(1-7). Additionally, leptin antagonist also markedly suppressed the overexpressions of p38 and ERK1/2 induced by HG, whereas leptin antagonist had no influence on the overexpression of c-Jun N-terminal kinase. More remarkable, Ang-(1-7), leptin antagonist, SB203580 or SP600125, respectively, significantly inhibited the injuries induced by HG, such as the increased cell viability, decreased apoptotic rate, reduction of ROS production and increased mitochondrial membrane potential. Furthermore, the overexpressions of p38 MAPK, ERK1/2 and leptin were suppressed by N-actyl-L-cystine.
Conclusions: The present findings show that Ang-(1-7) protects from HG-stimulated damage as an inhibitor of the reactive oxygen species-leptin-p38 MAPK/ERK1/2 pathways, but not the leptin-c-Jun N-terminal kinase pathway in vitro.
Keywords: Angiotensin-(1-7); Cardiomyocytes; High glucose.
© 2016 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures


Similar articles
-
Naringin inhibits ROS-activated MAPK pathway in high glucose-induced injuries in H9c2 cardiac cells.Basic Clin Pharmacol Toxicol. 2014 Apr;114(4):293-304. doi: 10.1111/bcpt.12153. Epub 2013 Dec 11. Basic Clin Pharmacol Toxicol. 2014. PMID: 24118820
-
Exogenous hydrogen sulfide alleviates high glucose-induced cardiotoxicity via inhibition of leptin signaling in H9c2 cells.Mol Cell Biochem. 2014 Jun;391(1-2):147-55. doi: 10.1007/s11010-014-1997-3. Epub 2014 Apr 1. Mol Cell Biochem. 2014. PMID: 24687304
-
Protective effect of angiotensin-(1-7) against hyperglycaemia-induced injury in H9c2 cardiomyoblast cells via the PI3K̸Akt signaling pathway.Int J Mol Med. 2018 Mar;41(3):1283-1292. doi: 10.3892/ijmm.2017.3322. Epub 2017 Dec 15. Int J Mol Med. 2018. PMID: 29286068 Free PMC article.
-
Exogenous hydrogen sulfide protects H9c2 cardiac cells against high glucose-induced injury by inhibiting the activities of the p38 MAPK and ERK1/2 pathways.Int J Mol Med. 2013 Oct;32(4):917-25. doi: 10.3892/ijmm.2013.1462. Epub 2013 Aug 1. Int J Mol Med. 2013. PMID: 23912965
-
Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications.Front Endocrinol (Lausanne). 2019 Sep 18;10:640. doi: 10.3389/fendo.2019.00640. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31620092 Free PMC article. Review.
Cited by
-
MicroRNA-27b inhibition promotes Nrf2/ARE pathway activation and alleviates intracerebral hemorrhage-induced brain injury.Oncotarget. 2017 Aug 7;8(41):70669-70684. doi: 10.18632/oncotarget.19974. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050310 Free PMC article.
-
Paracrine and Intracrine Angiotensin 1-7/Mas Receptor Axis in the Substantia Nigra of Rodents, Monkeys, and Humans.Mol Neurobiol. 2018 Jul;55(7):5847-5867. doi: 10.1007/s12035-017-0805-y. Epub 2017 Oct 30. Mol Neurobiol. 2018. PMID: 29086247 Free PMC article.
-
Phosphocreatine Improves Cardiac Dysfunction by Normalizing Mitochondrial Respiratory Function through JAK2/STAT3 Signaling Pathway In Vivo and In Vitro.Oxid Med Cell Longev. 2019 Nov 30;2019:6521218. doi: 10.1155/2019/6521218. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885809 Free PMC article.
-
[Angiotensin-(1-7) protects cardiac myocytes against high glucose-induced injury by inhibiting ClC-3 chloride channels].Nan Fang Yi Ke Da Xue Xue Bao. 2017 Jul 20;37(7):895-901. doi: 10.3969/j.issn.1673-4254.2017.07.07. Nan Fang Yi Ke Da Xue Xue Bao. 2017. PMID: 28736364 Free PMC article. Chinese.
-
Mas receptor: a potential strategy in the management of ischemic cardiovascular diseases.Cell Cycle. 2023 Jul;22(13):1654-1674. doi: 10.1080/15384101.2023.2228089. Epub 2023 Jun 26. Cell Cycle. 2023. PMID: 37365840 Free PMC article. Review.
References
-
- Chen J, Mo H, Guo R, et al Inhibition of the leptin‐induced activation of the p38 MAPK pathway contributes to the protective effects of naringin against high glucose‐induced injury in H9c2 cardiac cells. Int J Mol Med 2014; 33: 605–612. - PubMed
-
- Majumdar P, Chen S, George B, et al Leptin and endothelin‐1 mediated increased extracellular matrix protein production and cardiomyocyte hypertrophy in diabetic heart disease. Diabetes Metab Res Rev 2009; 25: 452–463. - PubMed
-
- Evans JL, Goldfine ID, Maddux BA, et al Oxidative stress and stress‐activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002; 23: 599–622. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

