Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment
- PMID: 27895645
- PMCID: PMC5107568
- DOI: 10.3389/fimmu.2016.00502
Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment
Abstract
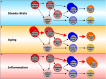
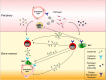
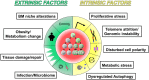
All hematopoietic and immune cells are continuously generated by hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) through highly organized process of stepwise lineage commitment. In the steady state, HSCs are mostly quiescent, while HPCs are actively proliferating and contributing to daily hematopoiesis. In response to hematopoietic challenges, e.g., life-threatening blood loss, infection, and inflammation, HSCs can be activated to proliferate and engage in blood formation. The HSC activation induced by hematopoietic demand is mediated by direct or indirect sensing mechanisms involving pattern recognition receptors or cytokine/chemokine receptors. In contrast to the hematopoietic challenges with obvious clinical symptoms, how the aging process, which involves low-grade chronic inflammation, impacts hematopoiesis remains undefined. Herein, we summarize recent findings pertaining to functional alternations of hematopoiesis, HSCs, and the bone marrow (BM) microenvironment during the processes of aging and inflammation and highlight some common cellular and molecular changes during the processes that influence hematopoiesis and its cells of origin, HSCs and HPCs, as well as the BM microenvironment. We also discuss how age-dependent alterations of the immune system lead to subclinical inflammatory states and how inflammatory signaling might be involved in hematopoietic aging. Our aim is to present evidence supporting the concept of "Inflamm-Aging," or inflammation-associated aging of hematopoiesis.
Keywords: ageing; cytokine; hematopoietic stem cell; inflammation; niche; pathogen recognition receptor.
Figures

Similar articles
-
Emerging insights into epigenetics and hematopoietic stem cell trafficking in age-related hematological malignancies.Stem Cell Res Ther. 2024 Nov 6;15(1):401. doi: 10.1186/s13287-024-04008-4. Stem Cell Res Ther. 2024. PMID: 39506818 Free PMC article. Review.
-
Protection of hematopoietic stem cells from stress-induced exhaustion and aging.Curr Opin Hematol. 2020 Jul;27(4):225-231. doi: 10.1097/MOH.0000000000000586. Curr Opin Hematol. 2020. PMID: 32398455 Review.
-
Parallels between immune driven-hematopoiesis and T cell activation: 3 signals that relay inflammatory stress to the bone marrow.Exp Cell Res. 2014 Dec 10;329(2):239-47. doi: 10.1016/j.yexcr.2014.09.016. Epub 2014 Sep 22. Exp Cell Res. 2014. PMID: 25246130 Review.
-
[Inflammation and early hematopoiesis].Rinsho Ketsueki. 2018;59(10):1955-1961. doi: 10.11406/rinketsu.59.1955. Rinsho Ketsueki. 2018. PMID: 30305497 Review. Japanese.
-
Inflammatory bone marrow microenvironment.Hematology Am Soc Hematol Educ Program. 2019 Dec 6;2019(1):294-302. doi: 10.1182/hematology.2019000045. Hematology Am Soc Hematol Educ Program. 2019. PMID: 31808897 Free PMC article. Review.
Cited by
-
Common Sources of Inflammation and Their Impact on Hematopoietic Stem Cell Biology.Curr Stem Cell Rep. 2020;6(3):96-107. doi: 10.1007/s40778-020-00177-z. Epub 2020 Aug 17. Curr Stem Cell Rep. 2020. PMID: 32837857 Free PMC article. Review.
-
Towards a Better Understanding of Cohesin Mutations in AML.Front Oncol. 2019 Sep 9;9:867. doi: 10.3389/fonc.2019.00867. eCollection 2019. Front Oncol. 2019. PMID: 31552185 Free PMC article. Review.
-
Inflammation and hematopoietic stem cells aging.Blood Sci. 2020 Nov 17;3(1):1-5. doi: 10.1097/BS9.0000000000000063. eCollection 2021 Jan. Blood Sci. 2020. PMID: 35399205 Free PMC article. Review.
-
Do haematopoietic stem cells age?Nat Rev Immunol. 2020 Mar;20(3):196-202. doi: 10.1038/s41577-019-0236-2. Epub 2019 Nov 18. Nat Rev Immunol. 2020. PMID: 31740804 Free PMC article. Review.
-
Inhibitor of DNA binding proteins revealed as orchestrators of steady state, stress and malignant hematopoiesis.Front Immunol. 2022 Aug 5;13:934624. doi: 10.3389/fimmu.2022.934624. eCollection 2022. Front Immunol. 2022. PMID: 35990659 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

