Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells
- PMID: 27895384
- PMCID: PMC5110938
- DOI: 10.3164/jcbn.16-26
Exendin-4 induces extracellular-superoxide dismutase through histone H3 acetylation in human retinal endothelial cells
Abstract
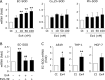
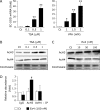
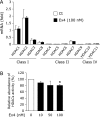
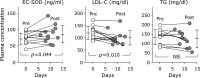
Extracellular-superoxide dismutase (genetic name SOD3) is a secreted anti-oxidative enzyme, and its presence in vascular walls may play an important role in protecting the vascular system against oxidative stress. Oxidative stress has been implicated in the pathogenesis of diabetic retinopathy; therefore, increases in extracellular-superoxide dismutase have been suggested to inhibit the progression of diabetic retinopathy. Incretin-based drugs such as glucagon-like peptide-1 receptor agonists are used in the treatment of type 2 diabetes. Glucagon-like peptide-1 receptor agonists are expected to function as extrapancreatic agents because the glucagon-like peptide-1 receptor is expressed not only in pancreatic tissues, but also in many other tissue types. We herein demonstrated that exendin-4, a glucagon-like peptide-1 receptor agonist, induced the expression of extracellular-superoxide dismutase in human retinal microvascular endothelial cells through epigenetic regulation. The results of the present study demonstrated that exendin-4 induced the expression of extracellular-superoxide dismutase through histone H3 acetylation at the SOD3 proximal promoter region. Moreover, plasma extracellular-superoxide dismutase concentrations in diabetic patients were elevated by incretin-based therapies. Therefore, incretin-based therapies may exert direct extrapancreatic effects in order to protect blood vessels by enhancing anti-oxidative activity.
Keywords: diabetic retinopathy; epigenetics; exendin-4; extracellular-superoxide dismutase; incretin-based therapy.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

Similar articles
-
CAPE increases the expression of SOD3 through epigenetics in human retinal endothelial cells.J Clin Biochem Nutr. 2017 Jul;61(1):6-13. doi: 10.3164/jcbn.16-109. Epub 2017 Jun 20. J Clin Biochem Nutr. 2017. PMID: 28751803 Free PMC article.
-
Exendin-4 promotes extracellular-superoxide dismutase expression in A549 cells through DNA demethylation.J Clin Biochem Nutr. 2016 Jan;58(1):34-9. doi: 10.3164/jcbn.15-16. Epub 2015 Nov 20. J Clin Biochem Nutr. 2016. PMID: 26798195 Free PMC article.
-
Regulation of Oxidative Stress in Pulmonary Artery Endothelium. Modulation of Extracellular Superoxide Dismutase and NOX4 Expression Using Histone Deacetylase Class I Inhibitors.Am J Respir Cell Mol Biol. 2015 Oct;53(4):513-24. doi: 10.1165/rcmb.2014-0260OC. Am J Respir Cell Mol Biol. 2015. PMID: 25749103 Free PMC article.
-
[Regulation of Extracellular Redox Homeostasis in Tumor Microenvironment].Yakugaku Zasshi. 2019;139(9):1139-1144. doi: 10.1248/yakushi.19-00128. Yakugaku Zasshi. 2019. PMID: 31474628 Review. Japanese.
-
Exendin-4 from Heloderma suspectum venom: From discovery to its latest application as type II diabetes combatant.Basic Clin Pharmacol Toxicol. 2019 May;124(5):513-527. doi: 10.1111/bcpt.13169. Epub 2018 Dec 12. Basic Clin Pharmacol Toxicol. 2019. PMID: 30417596 Review.
Cited by
-
Sodium Acetate Enhances Neutrophil Extracellular Trap Formation via Histone Acetylation Pathway in Neutrophil-like HL-60 Cells.Int J Mol Sci. 2024 Aug 11;25(16):8757. doi: 10.3390/ijms25168757. Int J Mol Sci. 2024. PMID: 39201443 Free PMC article.
-
Tumor necrosis factor-α decreases EC-SOD expression through DNA methylation.J Clin Biochem Nutr. 2017 May;60(3):169-175. doi: 10.3164/jcbn.16-111. Epub 2017 Apr 7. J Clin Biochem Nutr. 2017. PMID: 28584398 Free PMC article.
-
Epigenetic control of gene regulation during development and disease: A view from the retina.Prog Retin Eye Res. 2018 Jul;65:1-27. doi: 10.1016/j.preteyeres.2018.03.002. Epub 2018 Mar 12. Prog Retin Eye Res. 2018. PMID: 29544768 Free PMC article. Review.
-
CAPE increases the expression of SOD3 through epigenetics in human retinal endothelial cells.J Clin Biochem Nutr. 2017 Jul;61(1):6-13. doi: 10.3164/jcbn.16-109. Epub 2017 Jun 20. J Clin Biochem Nutr. 2017. PMID: 28751803 Free PMC article.
-
Targeting epigenetic mechanisms in diabetic wound healing.Transl Res. 2019 Feb;204:39-50. doi: 10.1016/j.trsl.2018.10.001. Epub 2018 Oct 10. Transl Res. 2019. PMID: 30392877 Free PMC article. Review.
References
-
- Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334. - PubMed
-
- Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–3238. - PubMed
-
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, ; Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. - PubMed
-
- Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

