Activation of chronic toxoplasmosis by transportation stress in a mouse model
- PMID: 27895319
- PMCID: PMC5349993
- DOI: 10.18632/oncotarget.13568
Activation of chronic toxoplasmosis by transportation stress in a mouse model
Abstract
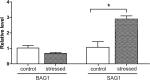
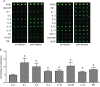
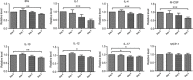
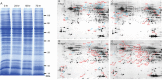
Toxoplasma gondii is an obligate intracellular parasite infecting 25% of the world population and enormous number of animals. It can exist in two forms in intermediate hosts: the fast replicating tachyzoites responsible for acute infection and the slowly replicating bradyzoites responsible for life-long chronic infection. The interconversion between tachyzoites and bradyzoites plays critical roles in the transmission and pathogenesis of T. gondii. However, the molecular mechanisms that govern the interconversion are largely unknown. In this study, we established a chronic infection model in mice and examined the impact of transportation stress on the status of chronic infection. Our results demonstrated that, treating chronically infected mice with conditions mimicking transportation stress reduced the levels of several key cytokines that restrict the infection at chronic stage. Increased expression of the tachyzoite specific gene SAG1 (surface antigen 1) was detected in brain cysts of stress treated mice, indicating activation and conversion of bradyzoites to tachyzoites. Using this model, we identified fifteen toxoplasmic proteins that had significant abundance changes during stress induced cysts reactivation. These proteins serve as a basis for further investigation of the mechanisms governing bradyzoite conversion.
Keywords: Toxoplasma gondii; bradyzoite; chronic infection; reactivation; transportation stress.
Conflict of interest statement
The authors declared that they have no conflicts of interest.
Figures
Similar articles
-
Real-time RT-PCR on SAG1 and BAG1 gene expression during stage conversion in immunosuppressed mice infected with Toxoplasma gondii Tehran strain.Korean J Parasitol. 2012 Sep;50(3):199-205. doi: 10.3347/kjp.2012.50.3.199. Epub 2012 Aug 13. Korean J Parasitol. 2012. PMID: 22949746 Free PMC article.
-
The determinants regulating Toxoplasma gondii bradyzoite development.Front Microbiol. 2022 Nov 11;13:1027073. doi: 10.3389/fmicb.2022.1027073. eCollection 2022. Front Microbiol. 2022. PMID: 36439853 Free PMC article. Review.
-
Primary culture of skeletal muscle cells as a model for studies of Toxoplasma gondii cystogenesis.J Parasitol. 2008 Feb;94(1):72-83. doi: 10.1645/GE-1273.1. J Parasitol. 2008. PMID: 18372624
-
Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host.Int J Parasitol. 2004 Mar 9;34(3):347-60. doi: 10.1016/j.ijpara.2003.11.024. Int J Parasitol. 2004. PMID: 15003495
-
Pathogeny and immunological control of toxoplasmosis.Braz J Med Biol Res. 1992;25(12):1163-9. Braz J Med Biol Res. 1992. PMID: 1341911 Review.
Cited by
-
Sixty Years (1957-2017) of Research on Toxoplasmosis in China-An Overview.Front Microbiol. 2017 Sep 25;8:1825. doi: 10.3389/fmicb.2017.01825. eCollection 2017. Front Microbiol. 2017. PMID: 28993763 Free PMC article. Review.
-
Toxoplasma gondii infection in people with schizophrenia is related to higher hair glucocorticoid levels.Front Psychiatry. 2024 Feb 16;15:1286135. doi: 10.3389/fpsyt.2024.1286135. eCollection 2024. Front Psychiatry. 2024. PMID: 38435971 Free PMC article.
-
Association between Toxoplasma gondii seropositivity and memory function in nondemented older adults.Neurobiol Aging. 2017 May;53:76-82. doi: 10.1016/j.neurobiolaging.2017.01.018. Epub 2017 Feb 3. Neurobiol Aging. 2017. PMID: 28235681 Free PMC article.
-
ANK1 and DnaK-TPR, Two Tetratricopeptide Repeat-Containing Proteins Primarily Expressed in Toxoplasma Bradyzoites, Do Not Contribute to Bradyzoite Differentiation.Front Microbiol. 2017 Nov 13;8:2210. doi: 10.3389/fmicb.2017.02210. eCollection 2017. Front Microbiol. 2017. PMID: 29180989 Free PMC article.
-
Contrasting Disease Progression, Microglia Reactivity, Tolerance, and Resistance to Toxoplasma gondii Infection in Two Mouse Strains.Biomedicines. 2024 Jun 26;12(7):1420. doi: 10.3390/biomedicines12071420. Biomedicines. 2024. PMID: 39061995 Free PMC article.
References
-
- Hill DE, Dubey JP. Toxoplasma gondii prevalence in farm animals in the United States. International journal for parasitology. 2013;43:107–113. - PubMed
-
- Dabritz HA, Conrad PA. Cats and Toxoplasma: implications for public health. Zoonoses and public health. 2010;57:34–52. - PubMed
-
- Gardner IA, Greiner M, Dubey JP. Statistical evaluation of test accuracy studies for Toxoplasma gondii in food animal intermediate hosts. Zoonoses and public health. 2010;57:82–94. - PubMed
-
- Jones JL, Dubey JP. Foodborne toxoplasmosis. Clinical infectious diseases. 2012;55:845–851. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

