Prion-Like Polymerization in Immunity and Inflammation
- PMID: 27881448
- PMCID: PMC5378051
- DOI: 10.1101/cshperspect.a023580
Prion-Like Polymerization in Immunity and Inflammation
Abstract
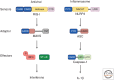
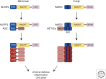
The innate immune system relies on receptors that sense common signs of infection to trigger a robust host-defense response. Receptors such as RIG-I and NLRP3 activate downstream adaptors mitochondrial antiviral signaling (MAVS) and apoptosis-associated speck-like protein (ASC), respectively, to propagate immune and inflammatory signaling. Recent studies have indicated that both MAVS and ASC form functional prion-like polymers to propagate immune signaling. Here, we summarize the biochemical, genetic, and structural studies that characterize the prion-like behavior of MAVS and ASC in their respective signaling pathways. We then discuss prion-like polymerization as an evolutionarily conserved mechanism of signal transduction in innate immunity in light of the similarity between the NLRP3-ASC, the NLRP3-ASC pathway in mammals, and the NWD2-HET-s pathway in fungi. We conclude by outlining the unique advantages to signaling through functional prions and potential future directions in the field.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

Similar articles
-
Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation.Cell. 2014 Mar 13;156(6):1207-1222. doi: 10.1016/j.cell.2014.01.063. Cell. 2014. PMID: 24630723 Free PMC article.
-
Prion-like polymerization as a signaling mechanism.Trends Immunol. 2014 Dec;35(12):622-630. doi: 10.1016/j.it.2014.10.003. Epub 2014 Nov 12. Trends Immunol. 2014. PMID: 25457352 Free PMC article. Review.
-
Partial Prion Cross-Seeding between Fungal and Mammalian Amyloid Signaling Motifs.mBio. 2021 Feb 9;12(1):e02782-20. doi: 10.1128/mBio.02782-20. mBio. 2021. PMID: 33563842 Free PMC article.
-
Prions and Prion-like assemblies in neurodegeneration and immunity: The emergence of universal mechanisms across health and disease.Semin Cell Dev Biol. 2020 Mar;99:115-130. doi: 10.1016/j.semcdb.2019.11.012. Epub 2019 Dec 6. Semin Cell Dev Biol. 2020. PMID: 31818518 Review.
-
Signal transduction by a fungal NOD-like receptor based on propagation of a prion amyloid fold.PLoS Biol. 2015 Feb 11;13(2):e1002059. doi: 10.1371/journal.pbio.1002059. eCollection 2015 Feb. PLoS Biol. 2015. PMID: 25671553 Free PMC article.
Cited by
-
Inflammasome proteins as biomarkers of traumatic brain injury.PLoS One. 2018 Dec 31;13(12):e0210128. doi: 10.1371/journal.pone.0210128. eCollection 2018. PLoS One. 2018. PMID: 30596792 Free PMC article. Clinical Trial.
-
Understand the Functions of Scaffold Proteins in Cell Signaling by a Mesoscopic Simulation Method.Biophys J. 2020 Nov 17;119(10):2116-2126. doi: 10.1016/j.bpj.2020.10.002. Epub 2020 Oct 14. Biophys J. 2020. PMID: 33113350 Free PMC article.
-
TIA-1 Is a Functional Prion-Like Protein.Cold Spring Harb Perspect Biol. 2017 May 1;9(5):a030718. doi: 10.1101/cshperspect.a030718. Cold Spring Harb Perspect Biol. 2017. PMID: 28003185 Free PMC article. Review.
-
Identification of metabolism related biomarkers in obesity based on adipose bioinformatics and machine learning.J Transl Med. 2024 Oct 31;22(1):986. doi: 10.1186/s12967-024-05615-8. J Transl Med. 2024. PMID: 39482740 Free PMC article.
-
PINK1 Inhibits Multimeric Aggregation and Signaling of MAVS and MAVS-Dependent Lung Pathology.Am J Respir Cell Mol Biol. 2021 May;64(5):592-603. doi: 10.1165/rcmb.2020-0490OC. Am J Respir Cell Mol Biol. 2021. PMID: 33577398 Free PMC article.
References
-
- Alberti S, Halfmann R, Lindquist S. 2010. Biochemical, cell biological, and genetic assays to analyze amyloid and prion aggregation in yeast. Methods Enzymol 470: 709–734. - PubMed
-
- Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, Antón J, et al. 2014. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15: 738–748. - PubMed
-
- Biswas SK, Lopez-Collazo E. 2009. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol 30: 475–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
