Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China
- PMID: 27874311
- PMCID: PMC5404380
- DOI: 10.1080/21645515.2016.1250990
Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China
Abstract
Objectives: To evaluate the persistence of protection from hepatitis B (HB) vaccination among adolescents immunized with a primary series of HB vaccine as infants, and the immune response to booster doses.
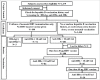
Methods: Healthy adolescents aged 15-17 y vaccinated with HB vaccine only at birth were enrolled. Baseline serum hepatitis B surface antigen (HBsAg), antibody against hepatitis B surface antigen (anti-HBs) and antibody against hepatitis B core antigen (anti-HBc) were detected by Enzyme-Linked Immunosorbent Assay (ELISA) and anti-HBs level was measured using Chemiluminescent Microparticle Immunoassay (CMIA). The rate of HBV infection was calculated. The seroprotection rate of anti-HBs (≥ 10 mIU/ml) and GMC level were used to evaluate the persistence of immunity from HB vaccination. Those with anti-HBs < 10 mIU/ml were immunized with booster doses of HB vaccine and the anamnestic response was assessed.
Results: Of 180 adolescents who received a primary series of HB vaccinations as infants, 3 (1.7%) had HBV infection and 74 (41.1%) had anti-HBs ≥ 10 mIU/ml with a GMC of 145.11 mIU/ml. The remaining 103 (57.2%) with anti-HBs < 10 mIU/ml received a booster dose of 20 μg HB vaccine and achieved the seroprotection rate of 84% (84/100) and a GMC of 875.19 mIU/ml at one month post-booster. An additional dose of 60 μg HB vaccine was administered to the 16 adolescents with anti-HBs < 10 mIU/ml after the first booster. All of them obtained anti-HBs seroprotection with a GMC of 271.02 mIU/ml at 1.5 months after an additional dose.
Conclusions: Vaccine-induced immunity persisted for up to 15-17 y in 89.3% (158/177) of participants after a primary HB vaccination in infancy. Administering a booster dose of 20μg HB vaccine elicited an anamnestic immune responses in the majority of individuals with baseline anti-HBs <10 mIU/ml.
Keywords: adolescents; booster vaccination; hepatitis B vaccine; immune memory; long-term immunogenicity.
Figures
Similar articles
-
Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: a 15-year follow-up study.Pediatr Infect Dis J. 2008 Oct;27(10):881-5. doi: 10.1097/INF.0b013e31817702ba. Pediatr Infect Dis J. 2008. PMID: 18756185
-
Significance and anamnestic response in isolated hepatitis B core antibody-positive individuals 18 years after neonatal hepatitis B virus vaccination in Taiwan.Vaccine. 2012 Jun 8;30(27):4034-9. doi: 10.1016/j.vaccine.2012.04.031. Epub 2012 Apr 22. Vaccine. 2012. PMID: 22531558
-
[Anti-HBs persistence following primary vaccination with three doses of hepatitis B vaccine among normal and high-responder adults: a 3-year follow-up study].Zhonghua Yu Fang Yi Xue Za Zhi. 2016 Jun;50(6):478-83. doi: 10.3760/cma.j.issn.0253-9624.2016.06.002. Zhonghua Yu Fang Yi Xue Za Zhi. 2016. PMID: 27256725 Chinese.
-
Booster dose vaccination for preventing hepatitis B.Cochrane Database Syst Rev. 2016 Jun 7;2016(6):CD008256. doi: 10.1002/14651858.CD008256.pub3. Cochrane Database Syst Rev. 2016. PMID: 27271960 Free PMC article. Review.
-
Impact of maternal carrier status on immunologic markers for protection after hepatitis B vaccination in infancy: a meta-analysis.Vaccine. 2012 Sep 28;30(44):6314-26. doi: 10.1016/j.vaccine.2012.07.080. Epub 2012 Aug 8. Vaccine. 2012. PMID: 22885275 Review.
Cited by
-
Hepatitis B burden and population immunity in a high endemicity city - a geographically random household epidemiology study for evaluating achievability of elimination.Epidemiol Infect. 2023 Jan 11;151:e22. doi: 10.1017/S095026882300002X. Epidemiol Infect. 2023. PMID: 36628568 Free PMC article.
-
Insights into induction of the immune response by the hepatitis B vaccine.World J Gastroenterol. 2022 Aug 21;28(31):4249-4262. doi: 10.3748/wjg.v28.i31.4249. World J Gastroenterol. 2022. PMID: 36159002 Free PMC article. Review.
-
Complementary Presence of HBV Humoral and T-cell Response Provides Protective Immunity after Neonatal Immunization.J Clin Transl Hepatol. 2022 Aug 28;10(4):660-668. doi: 10.14218/JCTH.2021.00272. Epub 2022 Jan 4. J Clin Transl Hepatol. 2022. PMID: 36062290 Free PMC article.
-
Modeling long-term persistence after 8 years of hepatitis B booster vaccination in 5- to 15-year-old children.Hum Vaccin Immunother. 2022 Nov 30;18(5):2061247. doi: 10.1080/21645515.2022.2061247. Epub 2022 May 4. Hum Vaccin Immunother. 2022. PMID: 35507912 Free PMC article.
-
HBV Seroprotection and Anamnestic Response to Booster Vaccination in Pediatric Cancer Survivors.Glob Pediatr Health. 2021 Jul 14;8:2333794X211033452. doi: 10.1177/2333794X211033452. eCollection 2021. Glob Pediatr Health. 2021. PMID: 34350309 Free PMC article.
References
-
- WHO Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection Geneva: WHO, 2015. - PubMed
-
- Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl) 2009; 122:98-102; PMID:19187625 - PubMed
-
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al.. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis 2009; 200:39-47; PMID:19469708; http://dx.doi.org/10.1086/599332 - DOI - PubMed
-
- Chinese Academy of Medical Sciences, CAMS Chinese medical science and technology development reports Beijing: Science Publishing House, 2016.
-
- National HBV serosurvey for Chinese population aged 1–29 years China medical tribune. August 06, 2015; Sect D-01.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical