Immunomodulatory Function of the Tumor Suppressor p53 in Host Immune Response and the Tumor Microenvironment
- PMID: 27869779
- PMCID: PMC5133937
- DOI: 10.3390/ijms17111942
Immunomodulatory Function of the Tumor Suppressor p53 in Host Immune Response and the Tumor Microenvironment
Abstract
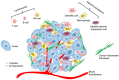
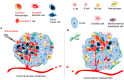
The tumor suppressor p53 is the most frequently mutated gene in human cancers. Most of the mutations are missense leading to loss of p53 function in inducing apoptosis and senescence. In addition to these autonomous effects of p53 inactivation/dysfunction on tumorigenesis, compelling evidence suggests that p53 mutation/inactivation also leads to gain-of-function or activation of non-autonomous pathways, which either directly or indirectly promote tumorigenesis. Experimental and clinical results suggest that p53 dysfunction fuels pro-tumor inflammation and serves as an immunological gain-of-function driver of tumorigenesis via skewing immune landscape of the tumor microenvironment (TME). It is now increasingly appreciated that p53 dysfunction in various cellular compartments of the TME leads to immunosuppression and immune evasion. Although our understanding of the cellular and molecular processes that link p53 activity to host immune regulation is still incomplete, it is clear that activating/reactivating the p53 pathway in the TME also represents a compelling immunological strategy to reverse immunosuppression and enhance antitumor immunity. Here, we review our current understanding of the potential cellular and molecular mechanisms by which p53 participates in immune regulation and discuss how targeting the p53 pathway can be exploited to alter the immunological landscape of tumors for maximizing therapeutic outcome.
Keywords: adaptive antitumor immunity; immune suppression; immunotherapy; inflammation; innate immunity; p53 inactivation; tumor microenvironment; tumor suppressor p53.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Local Activation of p53 in the Tumor Microenvironment Overcomes Immune Suppression and Enhances Antitumor Immunity.Cancer Res. 2017 May 1;77(9):2292-2305. doi: 10.1158/0008-5472.CAN-16-2832. Epub 2017 Mar 9. Cancer Res. 2017. PMID: 28280037 Free PMC article.
-
MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment.J Immunother Cancer. 2019 Nov 28;7(1):327. doi: 10.1186/s40425-019-0750-6. J Immunother Cancer. 2019. PMID: 31779710 Free PMC article.
-
New perspective on targeting the tumor suppressor p53 pathway in the tumor microenvironment to enhance the efficacy of immunotherapy.J Immunother Cancer. 2015 Mar 24;3:9. doi: 10.1186/s40425-015-0053-5. eCollection 2015. J Immunother Cancer. 2015. PMID: 25806108 Free PMC article.
-
[In-vivo activation of the p53 pathway by small-molecule antagonists of MDM2].Tanpakushitsu Kakusan Koso. 2007 Oct;52(13 Suppl):1816-7. Tanpakushitsu Kakusan Koso. 2007. PMID: 18051440 Review. Japanese. No abstract available.
-
[NEGATIVE REGULATORS OF TUMOR SUPPRESSOR P53 IN THE CONTEXT OF ANTICANCER THERAPY].Tsitologiia. 2015;57(12):847-54. Tsitologiia. 2015. PMID: 26995961 Review. Russian.
Cited by
-
Analysis of cancer-associated fibroblasts in cervical cancer by single-cell RNA sequencing.Aging (Albany NY). 2023 Dec 28;15(24):15340-15359. doi: 10.18632/aging.205353. Epub 2023 Dec 28. Aging (Albany NY). 2023. PMID: 38157264 Free PMC article.
-
TP53 Mutation-Mediated Immune Evasion in Cancer: Mechanisms and Therapeutic Implications.Cancers (Basel). 2024 Sep 3;16(17):3069. doi: 10.3390/cancers16173069. Cancers (Basel). 2024. PMID: 39272927 Free PMC article. Review.
-
Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy.Nat Commun. 2022 Feb 9;13(1):758. doi: 10.1038/s41467-022-28279-8. Nat Commun. 2022. PMID: 35140208 Free PMC article.
-
Differential p53-Mediated Cellular Responses to DNA-Damaging Therapeutic Agents.Int J Mol Sci. 2021 Oct 31;22(21):11828. doi: 10.3390/ijms222111828. Int J Mol Sci. 2021. PMID: 34769259 Free PMC article. Review.
-
Ras-p53 genomic cooperativity as a model to investigate mechanisms of innate immune regulation in gastrointestinal cancers.Oncotarget. 2021 Sep 28;12(20):2104-2110. doi: 10.18632/oncotarget.27983. eCollection 2021 Sep 28. Oncotarget. 2021. PMID: 34611484 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

