Protein Quality Control in Health and Disease
- PMID: 27864315
- PMCID: PMC5334259
- DOI: 10.1101/cshperspect.a023523
Protein Quality Control in Health and Disease
Abstract
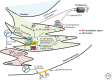
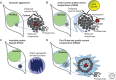
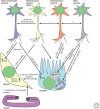
Maintaining functional protein homeostasis (proteostasis) is a constant challenge in the face of limited protein-folding capacity, environmental threats, and aging. Cells have developed several quality-control mechanisms that assist nascent polypeptides to fold properly, clear misfolded molecules, respond to the accumulation of protein aggregates, and deposit potentially toxic conformers in designated sites. Proteostasis collapse can lead to the development of diseases known as proteinopathies. Here we delineate the current knowledge on the different layers of protein quality-control mechanisms at the organelle and cellular levels with an emphasis on the prion protein (PrP). We also describe how protein quality control is integrated at the organismal level and discuss future perspectives on utilizing proteostasis maintenance as a strategy to develop novel therapies for the treatment of proteinopathies.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

Similar articles
-
Vesicle-mediated secretion of misfolded prion protein molecules from cyclosporin A-treated cells.FASEB J. 2018 Mar;32(3):1479-1492. doi: 10.1096/fj.201700598RRR. Epub 2018 Jan 3. FASEB J. 2018. PMID: 29127190
-
Underlying mechanisms and chemical/biochemical therapeutic approaches to ameliorate protein misfolding neurodegenerative diseases.Biofactors. 2017 Nov;43(6):737-759. doi: 10.1002/biof.1264. Epub 2016 Feb 22. Biofactors. 2017. PMID: 26899445 Review.
-
Regulating extracellular proteostasis capacity through the unfolded protein response.Prion. 2015;9(1):10-21. doi: 10.1080/19336896.2015.1011887. Prion. 2015. PMID: 25946012 Free PMC article.
-
Unfolded Protein Response and Macroautophagy in Alzheimer's, Parkinson's and Prion Diseases.Molecules. 2015 Dec 18;20(12):22718-56. doi: 10.3390/molecules201219865. Molecules. 2015. PMID: 26694349 Free PMC article. Review.
-
Proteostasis: bad news and good news from the endoplasmic reticulum.Swiss Med Wkly. 2014 Aug 21;144:w14001. doi: 10.4414/smw.2014.14001. eCollection 2014. Swiss Med Wkly. 2014. PMID: 25144910 Review.
Cited by
-
Cathepsin B promotes Aβ proteotoxicity by modulating aging regulating mechanisms.Nat Commun. 2024 Oct 3;15(1):8564. doi: 10.1038/s41467-024-52540-x. Nat Commun. 2024. PMID: 39362844 Free PMC article.
-
Renal tubular epithelial cell quality control mechanisms as therapeutic targets in renal fibrosis.J Pharm Anal. 2024 Aug;14(8):100933. doi: 10.1016/j.jpha.2024.01.001. Epub 2024 Jan 3. J Pharm Anal. 2024. PMID: 39247486 Free PMC article. Review.
-
Neuronal CBP-1 is Required for Enhanced Body Muscle Proteostasis in Response to Reduced Translation Downstream of mTOR.Front Biosci (Landmark Ed). 2024 Jul 23;29(7):264. doi: 10.31083/j.fbl2907264. Front Biosci (Landmark Ed). 2024. PMID: 39082355 Free PMC article.
-
Influenza A virus propagation requires the activation of the unfolded protein response and the accumulation of insoluble protein aggregates.iScience. 2024 Feb 2;27(3):109100. doi: 10.1016/j.isci.2024.109100. eCollection 2024 Mar 15. iScience. 2024. PMID: 38405606 Free PMC article.
-
Saccharomyces cerevisiae as a Model for Studying Human Neurodegenerative Disorders: Viral Capsid Protein Expression.Int J Mol Sci. 2023 Dec 7;24(24):17213. doi: 10.3390/ijms242417213. Int J Mol Sci. 2023. PMID: 38139041 Free PMC article. Review.
References
-
- Aguzzi A, Calella AM. 2009. Prions: Protein aggregation and infectious diseases. Physiol Rev 89: 1105–1152. - PubMed
-
- Amaducci L, Tesco G. 1994. Aging as a major risk for degenerative diseases of the central nervous system. Curr Opin Neurol 7: 283–286. - PubMed
-
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. 2004. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431: 805–810. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
