Mourning Dr. Alfred G. Knudson: the two-hit hypothesis, tumor suppressor genes, and the tuberous sclerosis complex
- PMID: 27862655
- PMCID: PMC5276834
- DOI: 10.1111/cas.13116
Mourning Dr. Alfred G. Knudson: the two-hit hypothesis, tumor suppressor genes, and the tuberous sclerosis complex
Abstract
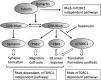
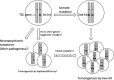
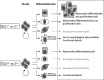
On July 10, 2016, Alfred G. Knudson, Jr., MD, PhD, a leader in cancer research, died at the age of 93 years. We deeply mourn his loss. Knudson's two-hit hypothesis, published in 1971, has been fundamental for understanding tumor suppressor genes and familial tumor-predisposing syndromes. To understand the molecular mechanism of two-hit-initiated tumorigenesis, Knudson used an animal model of a dominantly inherited tumor, the Eker rat. From the molecular identification of Tsc2 germline mutations, the Eker rat became a model for tuberous sclerosis complex (TSC), a familial tumor-predisposing syndrome. Animal models, including the fly, have greatly contributed to TSC research. Because the product of the TSC2/Tsc2 gene (tuberin) together with hamartin, the product of another TSC gene (TSC1/Tsc1), suppresses mammalian/mechanistic target of rapamycin complex 1 (mTORC1), rapalogs have been used as therapeutic drugs for TSC. Although significant activity of these drugs has been reported, there are still problems such as recurrence of residual tumors and adverse effects. Recent studies indicate that there are mTORC1-independent signaling pathways downstream of hamartin/tuberin, which may represent new therapeutic targets. The establishment of cellular models, such as pluripotent stem cells with TSC2/Tsc2 gene mutations, will facilitate the understanding of new aspects of TSC pathogenesis and the development of novel treatment options. In this review, we look back at the history of Knudson and animal models of TSC and introduce recent progress in TSC research.
Keywords: Eker rat; retinoblastoma; tuberous sclerosis complex; tumor suppressor gene; two-hit hypothesis.
© 2016 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
Structure of the Tuberous Sclerosis Complex 2 (TSC2) N Terminus Provides Insight into Complex Assembly and Tuberous Sclerosis Pathogenesis.J Biol Chem. 2016 Sep 16;291(38):20008-20. doi: 10.1074/jbc.M116.732446. Epub 2016 Aug 4. J Biol Chem. 2016. PMID: 27493206 Free PMC article.
-
Tuberous sclerosis complex and DNA repair.Adv Exp Med Biol. 2010;685:84-94. doi: 10.1007/978-1-4419-6448-9_8. Adv Exp Med Biol. 2010. PMID: 20687497 Review.
-
Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation.J Neuropathol Exp Neurol. 2004 Dec;63(12):1236-42. doi: 10.1093/jnen/63.12.1236. J Neuropathol Exp Neurol. 2004. PMID: 15624760
-
Tuberous sclerosis as an underlying basis for infantile spasm.Int Rev Neurobiol. 2002;49:315-32. doi: 10.1016/s0074-7742(02)49019-8. Int Rev Neurobiol. 2002. PMID: 12040899 Review.
-
Therapeutic targeting of cellular metabolism in cells with hyperactive mTORC1: a paradigm shift.Mol Cancer Res. 2015 Jan;13(1):3-8. doi: 10.1158/1541-7786.MCR-14-0343. Epub 2014 Oct 8. Mol Cancer Res. 2015. PMID: 25298408 Free PMC article. Review.
Cited by
-
The molecular genetics of PI3K/PTEN/AKT/mTOR pathway in the malformations of cortical development.Genes Dis. 2023 Jul 16;11(5):101021. doi: 10.1016/j.gendis.2023.04.041. eCollection 2024 Sep. Genes Dis. 2023. PMID: 39006182 Free PMC article. Review.
-
In commemoration of the 2018 Mataro Nagayo Prize: A road to early diagnosis and monitoring of asbestos-related mesothelioma.Cancer Sci. 2019 May;110(5):1518-1524. doi: 10.1111/cas.14001. Epub 2019 Apr 8. Cancer Sci. 2019. PMID: 30888083 Free PMC article. Review.
-
Genetically transitional disease: conceptual understanding and applicability to rheumatic disease.Nat Rev Rheumatol. 2024 May;20(5):301-310. doi: 10.1038/s41584-024-01086-9. Epub 2024 Feb 28. Nat Rev Rheumatol. 2024. PMID: 38418715 Review.
-
lncRNA-PCAT1 rs2632159 polymorphism could be a biomarker for colorectal cancer susceptibility.Biosci Rep. 2019 Jul 12;39(7):BSR20190708. doi: 10.1042/BSR20190708. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 31253700 Free PMC article.
-
Inferring Novel Tumor Suppressor Genes with a Protein-Protein Interaction Network and Network Diffusion Algorithms.Mol Ther Methods Clin Dev. 2018 Jun 21;10:57-67. doi: 10.1016/j.omtm.2018.06.007. eCollection 2018 Sep 21. Mol Ther Methods Clin Dev. 2018. PMID: 30069494 Free PMC article.
References
-
- Knudson AG. Cancer genetics through a personal retrospectroscope. Genes Chrom Cancer 2003; 38: 288–91. - PubMed
-
- Auerbach C. A possible case of delayed mutation in man. Ann Hum Genet 1956; 20: 266–9. - PubMed
-
- Friend SH, Bernards R, Rogelj S et al A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 1986; 323: 643–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

