Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial
- PMID: 27856432
- PMCID: PMC5530335
- DOI: 10.1136/annrheumdis-2016-210310
Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial
Abstract
Objectives: To compare efficacy and safety of sarilumab monotherapy with adalimumab monotherapy in patients with active rheumatoid arthritis (RA) who should not continue treatment with methotrexate (MTX) due to intolerance or inadequate response.
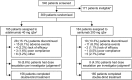
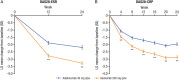
Methods: MONARCH was a randomised, active-controlled, double-blind, double-dummy, phase III superiority trial. Patients received sarilumab (200 mg every 2 weeks (q2w)) or adalimumab (40 mg q2w) monotherapy for 24 weeks. The primary end point was change from baseline in 28-joint disease activity score using erythrocyte sedimentation rate (DAS28-ESR) at week 24.
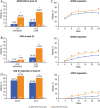
Results: Sarilumab was superior to adalimumab in the primary end point of change from baseline in DAS28-ESR (-3.28 vs -2.20; p<0.0001). Sarilumab-treated patients achieved significantly higher American College of Rheumatology 20/50/70 response rates (sarilumab: 71.7%/45.7%/23.4%; adalimumab: 58.4%/29.7%/11.9%; all p≤0.0074) and had significantly greater improvement in Health Assessment Questionnaire-Disability Index (p=0.0037). Importantly, at week 24, more patients receiving sarilumab compared with adalimumab achieved Clinical Disease Activity Index remission (7.1% vs 2.7%; nominal p=0.0468) and low disease activity (41.8% vs 24.9%; nominal p=0.0005, supplemental analysis). Adverse events occurred in 63.6% (adalimumab) and 64.1% (sarilumab) of patients, the most common being neutropenia and injection site reactions (sarilumab) and headache and worsening RA (adalimumab). Incidences of infections (sarilumab: 28.8%; adalimumab: 27.7%) and serious infections (1.1%, both groups) were similar, despite neutropenia differences.
Conclusions: Sarilumab monotherapy demonstrated superiority to adalimumab monotherapy by improving the signs and symptoms and physical functions in patients with RA who were unable to continue MTX treatment. The safety profiles of both therapies were consistent with anticipated class effects.
Trial registration number: NCT02332590.
Keywords: DMARDs (biologic); Rheumatoid Arthritis; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures
Similar articles
-
Safety and efficacy of switching from adalimumab to sarilumab in patients with rheumatoid arthritis in the ongoing MONARCH open-label extension.RMD Open. 2019 Oct 18;5(2):e001017. doi: 10.1136/rmdopen-2019-001017. eCollection 2019. RMD Open. 2019. PMID: 31673415 Free PMC article. Clinical Trial.
-
Patient-reported outcomes from a randomized phase III trial of sarilumab monotherapy versus adalimumab monotherapy in patients with rheumatoid arthritis.Arthritis Res Ther. 2018 Jun 19;20(1):129. doi: 10.1186/s13075-018-1614-z. Arthritis Res Ther. 2018. PMID: 29921318 Free PMC article. Clinical Trial.
-
Efficacy and safety of sarilumab in combination with csDMARDs or as monotherapy in subpopulations of patients with moderately to severely active rheumatoid arthritis in three phase III randomized, controlled studies.Arthritis Res Ther. 2020 Jun 10;22(1):139. doi: 10.1186/s13075-020-02194-z. Arthritis Res Ther. 2020. PMID: 32522251 Free PMC article. Clinical Trial.
-
Sarilumab: Review of a Second IL-6 Receptor Antagonist Indicated for the Treatment of Rheumatoid Arthritis.Ann Pharmacother. 2018 Aug;52(8):780-791. doi: 10.1177/1060028018761599. Epub 2018 Feb 26. Ann Pharmacother. 2018. PMID: 29482351 Review.
-
Evaluation of the efficacy and safety of sarilumab combination therapy in patients with rheumatoid arthritis with inadequate response to conventional disease-modifying antirheumatic drugs or tumour necrosis factor α inhibitors: systematic literature review and network meta-analyses.RMD Open. 2019 Feb 18;5(1):e000798. doi: 10.1136/rmdopen-2018-000798. eCollection 2019. RMD Open. 2019. PMID: 30886733 Free PMC article. Review.
Cited by
-
SARS-CoV-2: An Update on Potential Antivirals in Light of SARS-CoV Antiviral Drug Discoveries.Vaccines (Basel). 2020 Jun 23;8(2):335. doi: 10.3390/vaccines8020335. Vaccines (Basel). 2020. PMID: 32585913 Free PMC article. Review.
-
Safety and efficacy of switching from adalimumab to sarilumab in patients with rheumatoid arthritis in the ongoing MONARCH open-label extension.RMD Open. 2019 Oct 18;5(2):e001017. doi: 10.1136/rmdopen-2019-001017. eCollection 2019. RMD Open. 2019. PMID: 31673415 Free PMC article. Clinical Trial.
-
Indirect Treatment Comparison of the Efficacy and Safety of Sarilumab Monotherapy in Rheumatoid Arthritis Patients with Inadequate Response to Conventional Disease-Modifying Antirheumatic Drugs.Adv Ther. 2019 Apr;36(4):817-827. doi: 10.1007/s12325-019-00912-x. Epub 2019 Mar 12. Adv Ther. 2019. PMID: 30864105 Free PMC article.
-
Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a randomized, placebo-controlled phase III trial in Japan.Arthritis Res Ther. 2019 Mar 20;21(1):79. doi: 10.1186/s13075-019-1856-4. Arthritis Res Ther. 2019. PMID: 30894208 Free PMC article. Clinical Trial.
-
Sarilumab: First Global Approval.Drugs. 2017 Apr;77(6):705-712. doi: 10.1007/s40265-017-0724-2. Drugs. 2017. PMID: 28290137 Review.
References
-
- Keystone EC, Kavanaugh AF, Sharp JT, et al. . Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11. 10.1002/art.20217 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
