Effects of Asian Dust Particles on the Early-Stage Antigen-Induced Immune Response of Asthma in NC/Nga Mice
- PMID: 27854355
- PMCID: PMC5129354
- DOI: 10.3390/ijerph13111144
Effects of Asian Dust Particles on the Early-Stage Antigen-Induced Immune Response of Asthma in NC/Nga Mice
Abstract
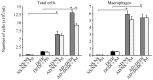
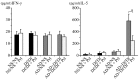
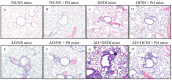
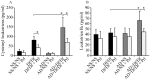
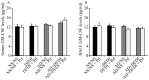
Asian dust (AD) can aggravate airway inflammation in asthma, but the association between AD and the development of asthma remains unclear. This study aimed to investigate the effects of AD on the early stage of antigen sensitization using a mouse model of asthma, as well as the role of leukotrienes (LTs) in antigen-induced airway inflammation potentiated by AD particles. NC/Nga mice were co-sensitized by intranasal instillation of AD particles and/or Dermatophagoides farinae (Df) for five consecutive days. Df-sensitized mice were stimulated with an intranasal Df challenge at seven days. Mice were treated with the type 1 cysteinyl LT (CysLT₁) receptor antagonist orally 4 h before and 1 h after the allergen challenge. At 24 h post-challenge, the differential leukocyte count, inflammatory cytokines, and LTs in bronchoalveolar lavage fluid were assessed, and airway inflammation was evaluated histopathologically. AD augmented neutrophilic and eosinophilic airway inflammation with increased CysLTs and dihydroxy-LT in a mouse model of asthma. The CysLT₁ receptor antagonist was shown to attenuate both neutrophilic and eosinophilic airway inflammation augmented by AD. Therefore, exposure to AD may be associated with the development of asthma and LTs may play important roles in airway inflammation augmented by AD.
Keywords: Asian dust; NC/Nga mouse model; airway inflammation; asthma; leukotrienes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Influence of Asian dust particles on immune adjuvant effects and airway inflammation in asthma model mice.PLoS One. 2014 Nov 11;9(11):e111831. doi: 10.1371/journal.pone.0111831. eCollection 2014. PLoS One. 2014. PMID: 25386753 Free PMC article.
-
Differential effects of dexamethasone and itraconazole on Aspergillus fumigatus-exacerbated allergic airway inflammation in a murine model of mite-sensitized asthma.Respiration. 2013;85(5):429-35. doi: 10.1159/000345861. Epub 2013 Jan 15. Respiration. 2013. PMID: 23327882
-
Intranasal mite allergen induces allergic asthma-like responses in NC/Nga mice.Life Sci. 2006 Jan 25;78(9):987-94. doi: 10.1016/j.lfs.2005.06.020. Epub 2005 Oct 17. Life Sci. 2006. PMID: 16229861
-
A review on leukotrienes and their receptors with reference to asthma.J Asthma. 2013 Nov;50(9):922-31. doi: 10.3109/02770903.2013.823447. Epub 2013 Aug 16. J Asthma. 2013. PMID: 23859232 Review.
-
Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling.J Allergy Clin Immunol. 2003 Jan;111(1 Suppl):S18-34; discussion S34-6. doi: 10.1067/mai.2003.25. J Allergy Clin Immunol. 2003. PMID: 12532084 Review.
Cited by
-
Inhale, exhale: Why particulate matter exposure in animal models are so acute? Data and facts behind the history.Data Brief. 2019 Jul 8;25:104237. doi: 10.1016/j.dib.2019.104237. eCollection 2019 Aug. Data Brief. 2019. PMID: 31367664 Free PMC article.
-
Respiratory and Systemic Toxicity of Inhaled Artificial Asian Sand Dust in Pigs.Life (Basel). 2021 Jan 4;11(1):25. doi: 10.3390/life11010025. Life (Basel). 2021. PMID: 33406620 Free PMC article.
References
-
- Zhu A., Ramanathan V., Li F., Kim D. Dust plumes over the Pacific, Indian, and Atlantic Oceans: Climatology and radiative impact. J. Geophys. Res.-Atmos. 2007 doi: 10.1029/2007JD008427. - DOI
-
- Tanaka T.Y., Chiba M. A numerical study of the contributions of dust source regions to the global dust budget. Glob. Planet Chang. 2006;52:88–104. doi: 10.1016/j.gloplacha.2006.02.002. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

