De-ubiquitinating enzyme, USP11, promotes transforming growth factor β-1 signaling through stabilization of transforming growth factor β receptor II
- PMID: 27853171
- PMCID: PMC5260874
- DOI: 10.1038/cddis.2016.371
De-ubiquitinating enzyme, USP11, promotes transforming growth factor β-1 signaling through stabilization of transforming growth factor β receptor II
Abstract
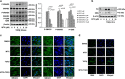
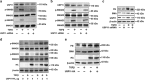
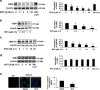
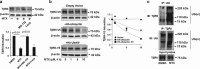
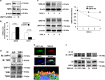
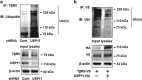
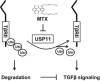
The transforming growth factor β-1 (TGFβ-1) signaling pathway plays a central role in the pathogenesis of pulmonary fibrosis. Two TGFβ-1 receptors, TβRI and TβRII, mediate this pathway. TβRI protein stability, as mediated by the ubiquitin/de-ubiquitination system, has been well studied; however, the molecular regulation of TβRII still remains unclear. Here we reveal that a de-ubiquitinating enzyme, USP11, promotes TGFβ-1 signaling through de-ubiquitination and stabilization of TβRII. We elucidate the role that mitoxantrone (MTX), an USP11 inhibitor, has in the attenuation of TGFβ-1 signaling. Inhibition or downregulation of USP11 results in increases in TβRII ubiquitination and reduction of TβRII stability. Subsequently, TGFβ-1 signaling is greatly attenuated, as shown by the decreases in phosphorylation of SMAD2/3 levels as well as that of fibronectin (FN) and smooth muscle actin (SMA). Overexpression of USP11 reduces TβRII ubiquitination and increases TβRII stabilization, thereby elevating phosphorylation of SMAD2/3 and the ultimate expression of FN and SMA. Further, elevated expression of USP11 and TβRII were detected in lung tissues from bleomycin-challenged mice and IPF patients. Therefore, USP11 may contribute to the pathogenesis of pulmonary fibrosis by stabilization of TβRII and promotion of TGFβ-1 signaling. This study provides mechanistic evidence for development of USP11 inhibitors as potential antifibrotic drugs for pulmonary fibrosis.
Figures

Similar articles
-
The inhibitory effect of ginsan on TGF-β mediated fibrotic process.J Cell Physiol. 2011 May;226(5):1241-7. doi: 10.1002/jcp.22452. J Cell Physiol. 2011. PMID: 20945375
-
USP7 Promotes TGF-β1 Signaling by De-Ubiquitinating Smad2/Smad3 in Pulmonary Fibrosis.Discov Med. 2024 Aug;36(187):1616-1626. doi: 10.24976/Discov.Med.202436187.148. Discov Med. 2024. PMID: 39190377
-
Ubiquitin-specific peptidase 11 promotes development of keloid derived fibroblasts by de-ubiquitinating TGF-β receptorII.Burns. 2024 Apr;50(3):641-652. doi: 10.1016/j.burns.2023.09.022. Epub 2023 Oct 6. Burns. 2024. PMID: 38097445
-
Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation.Cell Signal. 2013 Oct;25(10):2017-24. doi: 10.1016/j.cellsig.2013.06.001. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770288 Review.
-
TGF‑β/Smad signaling in chronic kidney disease: Exploring post‑translational regulatory perspectives (Review).Mol Med Rep. 2024 Aug;30(2):143. doi: 10.3892/mmr.2024.13267. Epub 2024 Jun 21. Mol Med Rep. 2024. PMID: 38904198 Free PMC article. Review.
Cited by
-
The Dual Role of USP11 in Cancer.J Oncol. 2022 Mar 22;2022:9963905. doi: 10.1155/2022/9963905. eCollection 2022. J Oncol. 2022. PMID: 35359344 Free PMC article. Review.
-
Regulation of XPC deubiquitination by USP11 in repair of UV-induced DNA damage.Oncotarget. 2017 Oct 29;8(57):96522-96535. doi: 10.18632/oncotarget.22105. eCollection 2017 Nov 14. Oncotarget. 2017. PMID: 29228550 Free PMC article.
-
Demethyleneberberine Alleviates Pulmonary Fibrosis through Disruption of USP11 Deubiquitinating GREM1.Pharmaceuticals (Basel). 2024 Feb 22;17(3):279. doi: 10.3390/ph17030279. Pharmaceuticals (Basel). 2024. PMID: 38543064 Free PMC article.
-
USP11 facilitates colorectal cancer proliferation and metastasis by regulating IGF2BP3 stability.Am J Transl Res. 2021 Feb 15;13(2):480-496. eCollection 2021. Am J Transl Res. 2021. PMID: 33594305 Free PMC article.
-
USP11 acts as a histone deubiquitinase functioning in chromatin reorganization during DNA repair.Nucleic Acids Res. 2019 Oct 10;47(18):9721-9740. doi: 10.1093/nar/gkz726. Nucleic Acids Res. 2019. PMID: 31504778 Free PMC article.
References
-
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell 1992; 71: 1003–1014. - PubMed
-
- Wrana JL, Carcamo J, Attisano L, Cheifetz S, Zentella A, Lopez-Casillas F et al. The type II TGF-beta receptor signals diverse responses in cooperation with the type I receptor. Cold Spring Harb Symp Quant Biol 1992; 57: 81–86. - PubMed
-
- Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol 2002; 71: 731–740. - PubMed
-
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390: 465–471. - PubMed
-
- Kawabata M, Miyazono K. Signal transduction of the TGF-beta superfamily by Smad proteins. J Biochem 1999; 125: 9–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

