Hepatitis C Treatment Uptake among Patients Who Have Received Opioid Substitution Treatment: A Population-Based Study
- PMID: 27846264
- PMCID: PMC5112941
- DOI: 10.1371/journal.pone.0166451
Hepatitis C Treatment Uptake among Patients Who Have Received Opioid Substitution Treatment: A Population-Based Study
Abstract
Background and aims: There is limited data on hepatitis C (HCV) treatment uptake among people who inject drugs including individuals receiving opioid substitution treatment (OST). We aimed to calculate cumulative HCV treatment uptake, estimate annual treatment rates, and identify factors associated with HCV treatment among individuals who have received OST in Norway.
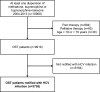
Methods: This observational study was based on linked data from The Norwegian Prescription Database and The Norwegian Surveillance System for Communicable Diseases between 2004 and 2013. Both registries have national coverage. From a total of 9919 individuals who had been dispensed OST (methadone, buprenorphine or buprenorphine-naloxone), we included 3755 individuals who had been notified with HCV infection. In this population, dispensions of HCV treatment (pegylated interferon and ribavirin), benzodiazepines, selective serotonin reuptake inhibitors and antipsychotics were studied.
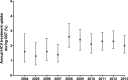
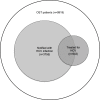
Results: Among 3755 OST patients notified with HCV infection, 539 (14%) had received HCV treatment during the study period. Annual HCV treatment rates during OST ranged between 1.3% (95% confidence interval [CI] 0.7-2.2) in 2005 and 2.6% (95% CI 1.9-3.5) in 2008 with no significant changes over time. HCV treatment uptake was not associated with age or gender, but associated with duration of active OST (adjusted odds ratio [aOR] 1.11 per year; 95% CI 1.07-1.15), high (> 80%) OST continuity (aOR 1.62; 95% CI 1.17-2.25), and heavy benzodiazepine use (aOR 0.65; 95% CI 0.49-0.87).
Conclusions: Cumulative HCV treatment uptake among OST patients notified with HCV infection in Norway between 2004 and 2013 was 14%. Annual treatment rates during OST remained unchanged below 3% per year. High continuity of OST over time and absence of heavy benzodiazepine use predicted HCV treatment uptake. Increased awareness for HCV among OST patients is needed as tolerable and efficient directly acting antiviral treatment is being introduced.
Conflict of interest statement
HM has held sponsored lectures for Roche, Medivir, Gilead Sciences, MSD and AbbVie. OD has received research grants from Gilead Sciences and MSD, been a consultant for Medivir, AbbVie, MSD and Bristol Myers Squibb, and held sponsored lectures for Medivir, Bristol Myers Squibb, Abbvie and Gilead. JGB, SS and JWH have declared no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Efficacy of response-guided directly observed pegylated interferon and self-administered ribavirin for people who inject drugs with hepatitis C virus genotype 2/3 infection: The ACTIVATE study.Int J Drug Policy. 2017 Sep;47:177-186. doi: 10.1016/j.drugpo.2017.05.020. Epub 2017 Jun 16. Int J Drug Policy. 2017. PMID: 28624134 Clinical Trial.
-
Adherence to response-guided pegylated interferon and ribavirin for people who inject drugs with hepatitis C virus genotype 2/3 infection: the ACTIVATE study.BMC Infect Dis. 2017 Jun 13;17(1):420. doi: 10.1186/s12879-017-2517-3. BMC Infect Dis. 2017. PMID: 28610605 Free PMC article. Clinical Trial.
-
Changes in risk behaviours during and following treatment for hepatitis C virus infection among people who inject drugs: The ACTIVATE study.Int J Drug Policy. 2017 Sep;47:230-238. doi: 10.1016/j.drugpo.2017.05.040. Epub 2017 Jun 19. Int J Drug Policy. 2017. PMID: 28633998 Clinical Trial.
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3 PMID: 28285495 Free PMC article. Updated. Review.
-
Hepatitis C treatment in patients with drug addiction: clinical management of interferon-alpha-associated psychiatric side effects.Curr Drug Abuse Rev. 2008 Jun;1(2):177-87. doi: 10.2174/1874473710801020177. Curr Drug Abuse Rev. 2008. PMID: 19630716 Review.
Cited by
-
On the path towards universal coverage of hepatitis C treatment among people receiving opioid agonist therapy (OAT) in Norway: a prospective cohort study from 2013 to 2017.BMJ Open. 2020 Aug 26;10(8):e036355. doi: 10.1136/bmjopen-2019-036355. BMJ Open. 2020. PMID: 32847908 Free PMC article.
-
High HCV cure rates among people who inject drugs and have suboptimal adherence: A patient-centered approach to HCV models of care.Int J Drug Policy. 2021 Jul;93:103135. doi: 10.1016/j.drugpo.2021.103135. Epub 2021 Mar 2. Int J Drug Policy. 2021. PMID: 33667826 Free PMC article.
-
Real-world hepatitis C treatment outcomes and reinfections among people who inject drugs at a needle and syringe program in Stockholm, Sweden.Harm Reduct J. 2023 Jun 12;20(1):72. doi: 10.1186/s12954-023-00801-1. Harm Reduct J. 2023. PMID: 37308951 Free PMC article.
-
Initiating HCV treatment with direct acting agents in opioid agonist treatment: When to start for people co-infected with HIV?Int J Drug Policy. 2017 Sep;47:169-176. doi: 10.1016/j.drugpo.2017.05.021. Epub 2017 Jun 1. Int J Drug Policy. 2017. PMID: 28578865 Free PMC article.
-
Uptake and predictors of direct-acting antiviral treatment for hepatitis C among people receiving opioid agonist therapy in Sweden and Norway: a drug utilization study from 2014 to 2017.Subst Abuse Treat Prev Policy. 2020 Jun 30;15(1):44. doi: 10.1186/s13011-020-00286-2. Subst Abuse Treat Prev Policy. 2020. PMID: 32605625 Free PMC article.
References
-
- Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. Journal of hepatology. 2014;61(1 Suppl):S45–57. - PubMed
-
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nature reviews Gastroenterology & hepatology. 2013;10(9):553–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

