Endoperoxide antimalarials: development, structural diversity and pharmacodynamic aspects with reference to 1,2,4-trioxane-based structural scaffold
- PMID: 27843298
- PMCID: PMC5098533
- DOI: 10.2147/DDDT.S118116
Endoperoxide antimalarials: development, structural diversity and pharmacodynamic aspects with reference to 1,2,4-trioxane-based structural scaffold
Abstract
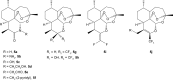
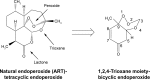
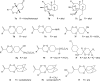
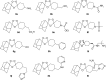
Malaria disease continues to be a major health problem worldwide due to the emergence of multidrug-resistant strains of Plasmodium falciparum. In recent days, artemisinin (ART)-based drugs and combination therapies remain the drugs of choice for resistant P. falciparum malaria. However, resistance to ART-based drugs has begun to appear in some parts of the world. Endoperoxide compounds (natural/semisynthetic/synthetic) representing a huge number of antimalarial agents possess a wide structural diversity with a desired antimalarial effectiveness against resistant P. falciparum malaria. The 1,2,4-trioxane ring system lacking the lactone ring that constitutes the most important endoperoxide structural scaffold is believed to be the key pharmacophoric moiety and is primarily responsible for the pharmacodynamic potential of endoperoxide-based antimalarials. Due to this reason, research into endoperoxide, particularly 1,2,4-trioxane-, 1,2,4-trioxolane- and 1,2,4,5-teraoxane-based scaffolds, has gained significant interest in recent years for developing antimalarial drugs against resistant malaria. In this paper, a comprehensive effort has been made to review the development of endoperoxide antimalarials from traditional antimalarial leads (natural/semisynthetic) and structural diversity of endoperoxide molecules derived from 1,2,4-trioxane-, 1,2,4-trioxolane- and 1,2,4,5-teraoxane-based structural scaffolds, including their chimeric (hybrid) molecules, which are newer and potent antimalarial agents.
Keywords: 1,2,4-trioxane; antimalarial; endoperoxide; pharmacodynamic; pharmacophore; structural diversity.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
An overview on the antimalarial activity of 1,2,4-trioxanes, 1,2,4-trioxolanes and 1,2,4,5-tetraoxanes.Med Res Rev. 2024 Jan;44(1):66-137. doi: 10.1002/med.21979. Epub 2023 May 24. Med Res Rev. 2024. PMID: 37222435
-
Enantiomeric 1,2,4-trioxanes display equivalent in vitro antimalarial activity versus Plasmodium falciparum malaria parasites: implications for the molecular mechanism of action of the artemisinins.Chembiochem. 2005 Nov;6(11):2048-54. doi: 10.1002/cbic.200500048. Chembiochem. 2005. PMID: 16222725
-
Synthesis and study of cytotoxic activity of 1,2,4-trioxane- and egonol-derived hybrid molecules against Plasmodium falciparum and multidrug-resistant human leukemia cells.Eur J Med Chem. 2014 Mar 21;75:403-12. doi: 10.1016/j.ejmech.2014.01.043. Epub 2014 Jan 31. Eur J Med Chem. 2014. PMID: 24561670
-
Exploration of artemisinin derivatives and synthetic peroxides in antimalarial drug discovery research.Eur J Med Chem. 2021 Mar 5;213:113193. doi: 10.1016/j.ejmech.2021.113193. Epub 2021 Jan 18. Eur J Med Chem. 2021. PMID: 33508479 Review.
-
Recent Developments in Natural Product Inspired Synthetic 1,2,4- Trioxolanes (Ozonides): An Unusual Entry into Antimalarial Chemotherapy.Curr Top Med Chem. 2019;19(10):831-846. doi: 10.2174/1568026619666190412104042. Curr Top Med Chem. 2019. PMID: 30977453 Review.
Cited by
-
The Antimalarial Drug Artesunate Mediates Selective Cytotoxicity by Upregulating HO-1 in Melanoma Cells.Biomedicines. 2023 Aug 27;11(9):2393. doi: 10.3390/biomedicines11092393. Biomedicines. 2023. PMID: 37760834 Free PMC article.
-
Cerebral Malaria and Neuronal Implications of Plasmodium Falciparum Infection: From Mechanisms to Advanced Models.Adv Sci (Weinh). 2022 Dec;9(36):e2202944. doi: 10.1002/advs.202202944. Epub 2022 Oct 27. Adv Sci (Weinh). 2022. PMID: 36300890 Free PMC article. Review.
-
Antileishmanial Anthracene Endoperoxides: Efficacy In Vitro, Mechanisms and Structure-Activity Relationships.Molecules. 2022 Oct 13;27(20):6846. doi: 10.3390/molecules27206846. Molecules. 2022. PMID: 36296439 Free PMC article.
-
Structural basis for endoperoxide-forming oxygenases.Beilstein J Org Chem. 2022 Jun 21;18:707-721. doi: 10.3762/bjoc.18.71. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 35821691 Free PMC article. Review.
-
Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy.Int J Mol Sci. 2022 May 25;23(11):5949. doi: 10.3390/ijms23115949. Int J Mol Sci. 2022. PMID: 35682626 Free PMC article. Review.
References
-
- WHO . World Malaria Report. Geneva: WHO; 2015.
-
- Roy S, Chetia D, Rudrapal M, Prakash A. Synthesis and antimalarial activity study of some new Mannich bases of 7-chloro-4-aminoquinoline. Med Chem. 2013;9(3):379–383. - PubMed
-
- Rudrapal M, Chetia D, Prakash A. Synthesis, antimalarial and antibacterial activity evaluation of some new 4-aminoquonoline derivatives. Med Chem Res. 2013;22(8):3703–3711.
-
- Warrell DA. Cerebral malaria. Schweiz Med Wochenschr. 1992;122(23):879–886. - PubMed
-
- Ridley RJ. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature. 2002;415:686–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

