Anti-fibrotic action of pirfenidone in Dupuytren's disease-derived fibroblasts
- PMID: 27835939
- PMCID: PMC5106805
- DOI: 10.1186/s12891-016-1326-y
Anti-fibrotic action of pirfenidone in Dupuytren's disease-derived fibroblasts
Abstract
Background: Dupuytren's disease (DD) is a complex fibro-proliferative disorder of the hand that is often progressive and eventually can cause contractures of the affected fingers. Transforming growth factor beta (TGF-β1) has been implicated as a key stimulator of myofibroblast activity and fascial contraction in DD. Pirfenidone (PFD) is an active small molecule shown to inhibit TGF-β1-mediated action in other fibrotic disorders. This study investigates the efficacy of PFD in vitro in inhibiting TGF-β1-mediated cellular functions leading to Dupuytren's fibrosis.
Methods: Fibroblasts harvested from (DD) and carpal tunnel (CT)- tissues were treated with or without TGF-β1 and/or PFD and were subjected to cell migration, cell proliferation and cell contraction assays. ELISA; western blots and real time RT-PCR assays were performed to determine the levels of fibronectin; p-Smad2/Smad3; alpha-smooth muscle actin (α-SMA), α2 chain of type I collagen and α1 chain of type III collagen respectively.
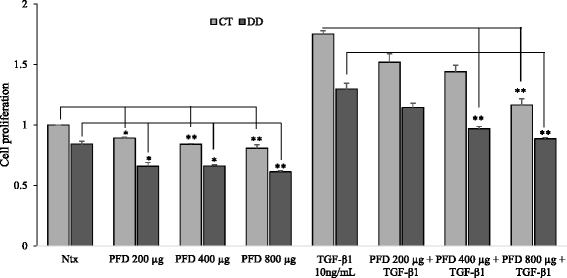
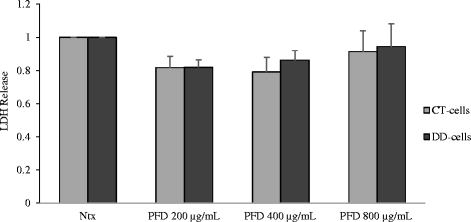
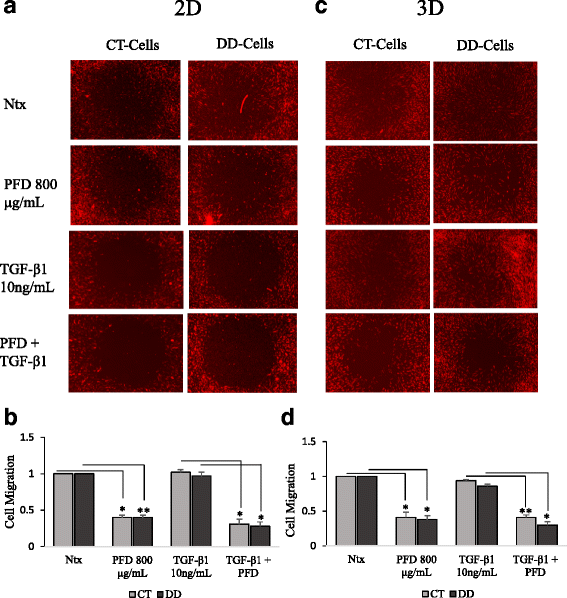
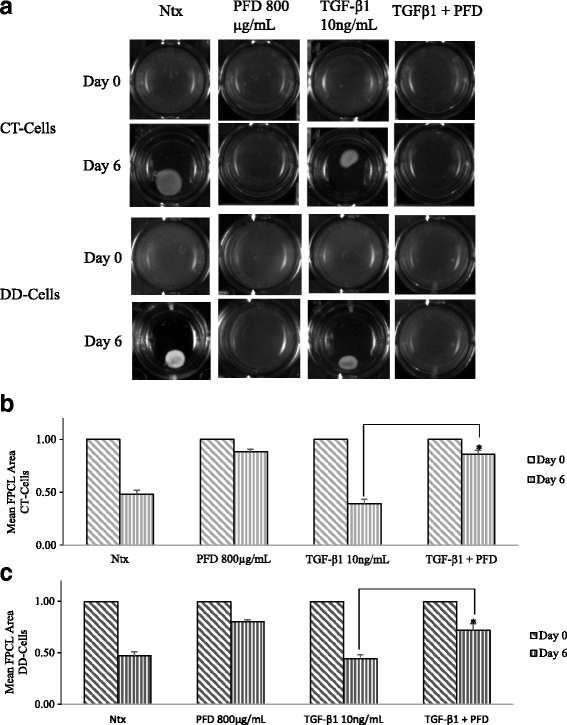
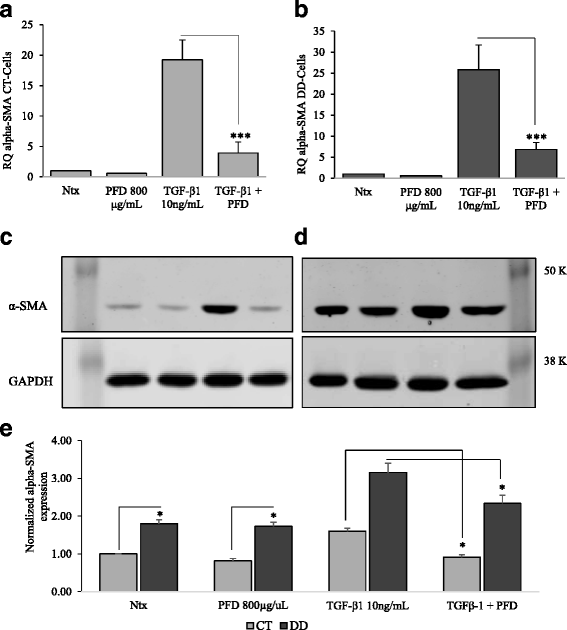
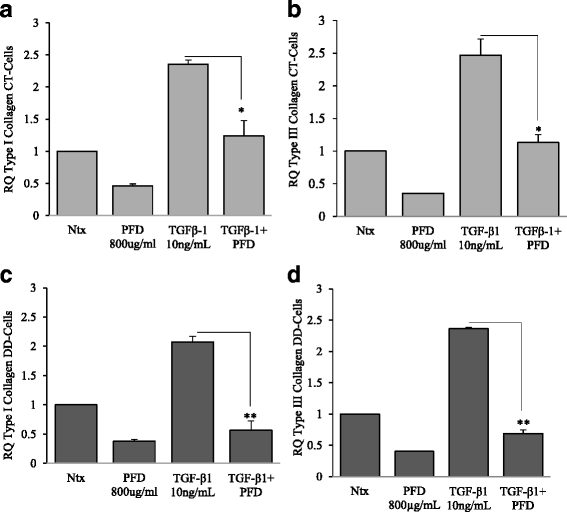
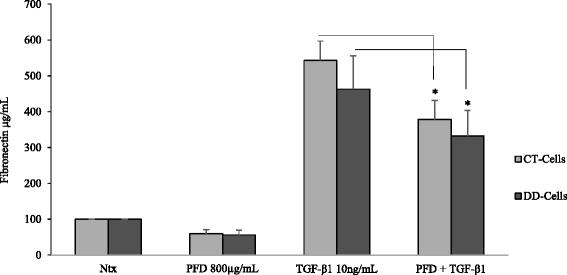
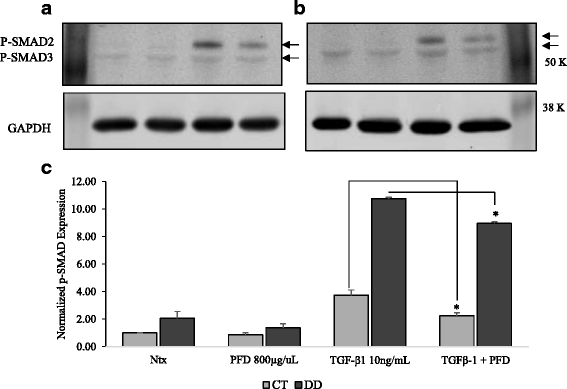
Results: Our results show that PFD effectively inhibits TGF-β1-induced cell migration, proliferation and cell contractile properties of both CT- and DD-derived fibroblasts. TGF-β1-induced α-SMA mRNA and protein levels were inhibited at the higher concentration of PFD (800 μg/ml). Interestingly, TGF-β1 induction of type I and type III collagens and fibronectin was inhibited by PFD in both CT- and DD- derived fibroblasts, but the effect was more prominent in DD cells. PFD down-regulated TGF-β1-induced phosphorylation of Smad2/Smad3, a key factor in the TGF-β1 signaling pathway.
Conclusion: Taken together these results suggest the PFD can potentially prevent TGF-β1-induced fibroblast to myofibroblast transformation and inhibit ECM production mainly Type I- and Type III- collagen and fibronectin in DD-derived fibroblasts. Further in-vivo studies with PFD may lead to a novel therapeutic application in preventing the progression or recurrence of Dupuytren's disease.
Keywords: Alpha-SMA; Carpal tunnel; Cell contraction; Cell migration; Collagen; Dupuytren’s contracture; Palmar fascia fibrosis; Smad2/Smad3.
Figures
Similar articles
-
Investigating the effects of Pirfenidone on TGF-β1 stimulated non-SMAD signaling pathways in Dupuytren's disease -derived fibroblasts.BMC Musculoskelet Disord. 2019 Mar 30;20(1):135. doi: 10.1186/s12891-019-2486-3. BMC Musculoskelet Disord. 2019. PMID: 30927912 Free PMC article.
-
Reversal of TGF-β1 stimulation of α-smooth muscle actin and extracellular matrix components by cyclic AMP in Dupuytren's-derived fibroblasts.BMC Musculoskelet Disord. 2011 May 25;12:113. doi: 10.1186/1471-2474-12-113. BMC Musculoskelet Disord. 2011. PMID: 21612641 Free PMC article.
-
[Tofacitinib inhibits the transformation of lung fibroblasts into myofibroblasts through JAK/STAT3 pathway].Beijing Da Xue Xue Bao Yi Xue Ban. 2024 Jun 18;56(3):505-511. doi: 10.19723/j.issn.1671-167X.2024.03.018. Beijing Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38864137 Free PMC article. Chinese.
-
Pirfenidone: Molecular Mechanisms and Potential Clinical Applications in Lung Disease.Am J Respir Cell Mol Biol. 2020 Apr;62(4):413-422. doi: 10.1165/rcmb.2019-0328TR. Am J Respir Cell Mol Biol. 2020. PMID: 31967851 Review.
-
Role and New Insights of Pirfenidone in Fibrotic Diseases.Int J Med Sci. 2015 Oct 14;12(11):840-7. doi: 10.7150/ijms.11579. eCollection 2015. Int J Med Sci. 2015. PMID: 26640402 Free PMC article. Review.
Cited by
-
Nanostring-Based Identification of the Gene Expression Profile in Trigger Finger Samples.Healthcare (Basel). 2021 Nov 20;9(11):1592. doi: 10.3390/healthcare9111592. Healthcare (Basel). 2021. PMID: 34828637 Free PMC article.
-
Current role of the collagenase Clostridium histolyticum in Dupuytren's disease treatment.Ir J Med Sci. 2020 May;189(2):529-534. doi: 10.1007/s11845-019-02127-z. Epub 2019 Nov 11. Ir J Med Sci. 2020. PMID: 31713028 Review.
-
The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing.Biomedicines. 2020 Apr 30;8(5):101. doi: 10.3390/biomedicines8050101. Biomedicines. 2020. PMID: 32365896 Free PMC article. Review.
-
Pharmacotherapies in Dupuytren Disease: Current and Novel Strategies.J Hand Surg Am. 2023 Aug;48(8):810-821. doi: 10.1016/j.jhsa.2023.02.003. Epub 2023 Mar 17. J Hand Surg Am. 2023. PMID: 36935324 Free PMC article. Review.
-
Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production.Blood. 2017 May 4;129(18):2570-2580. doi: 10.1182/blood-2017-01-758854. Epub 2017 Mar 2. Blood. 2017. PMID: 28254742 Free PMC article.
References
-
- Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24(1):153–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

