Towards an understanding of regulating Cajal body activity by protein modification
- PMID: 27819531
- PMCID: PMC5519237
- DOI: 10.1080/15476286.2016.1243649
Towards an understanding of regulating Cajal body activity by protein modification
Abstract
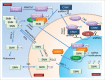
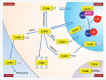
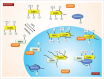
The biogenesis of small nuclear ribonucleoproteins (snRNPs), small Cajal body-specific RNPs (scaRNPs), small nucleolar RNPs (snoRNPs) and the telomerase RNP involves Cajal bodies (CBs). Although many components enriched in the CB contain post-translational modifications (PTMs), little is known about how these modifications impact individual protein function within the CB and, in concert with other modified factors, collectively regulate CB activity. Since all components of the CB also reside in other cellular locations, it is also important that we understand how PTMs affect the subcellular localization of CB components. In this review, we explore the current knowledge of PTMs on the activity of proteins known to enrich in CBs in an effort to highlight current progress as well as illuminate paths for future investigation.
Keywords: Cajal body; SMN; WRAP53; coilin; phosphorylation; post-translational modification; telomerase.
Figures
Similar articles
-
Identification of processing elements and interactors implicate SMN, coilin and the pseudogene-encoded coilp1 in telomerase and box C/D scaRNP biogenesis.RNA Biol. 2016 Oct 2;13(10):955-972. doi: 10.1080/15476286.2016.1211224. Epub 2016 Jul 15. RNA Biol. 2016. PMID: 27419845 Free PMC article.
-
Cajal body proteins differentially affect the processing of box C/D scaRNPs.PLoS One. 2015 Apr 13;10(4):e0122348. doi: 10.1371/journal.pone.0122348. eCollection 2015. PLoS One. 2015. PMID: 25875178 Free PMC article.
-
De novo formation of a subnuclear body.Science. 2008 Dec 12;322(5908):1713-7. doi: 10.1126/science.1165216. Epub 2008 Oct 23. Science. 2008. PMID: 18948503
-
Signals controlling Cajal body assembly and function.Int J Biochem Cell Biol. 2013 Jul;45(7):1314-7. doi: 10.1016/j.biocel.2013.03.019. Epub 2013 Apr 10. Int J Biochem Cell Biol. 2013. PMID: 23583661 Free PMC article. Review.
-
The Cajal body and the nucleolus: "In a relationship" or "It's complicated"?RNA Biol. 2017 Jun 3;14(6):739-751. doi: 10.1080/15476286.2016.1236169. Epub 2016 Sep 23. RNA Biol. 2017. PMID: 27661468 Free PMC article. Review.
Cited by
-
Coilin and Cajal bodies.Nucleus. 2023 Dec;14(1):2256036. doi: 10.1080/19491034.2023.2256036. Nucleus. 2023. PMID: 37682044 Free PMC article. Review.
-
The phospho-landscape of the survival of motoneuron protein (SMN) protein: relevance for spinal muscular atrophy (SMA).Cell Mol Life Sci. 2022 Aug 25;79(9):497. doi: 10.1007/s00018-022-04522-9. Cell Mol Life Sci. 2022. PMID: 36006469 Free PMC article. Review.
-
VRK1 functional insufficiency due to alterations in protein stability or kinase activity of human VRK1 pathogenic variants implicated in neuromotor syndromes.Sci Rep. 2019 Sep 16;9(1):13381. doi: 10.1038/s41598-019-49821-7. Sci Rep. 2019. PMID: 31527692 Free PMC article.
-
Post-Transcriptional and Post-Translational Modifications in Telomerase Biogenesis and Recruitment to Telomeres.Int J Mol Sci. 2023 Mar 6;24(5):5027. doi: 10.3390/ijms24055027. Int J Mol Sci. 2023. PMID: 36902458 Free PMC article. Review.
-
The Role of Telomerase and Telomeres in Interstitial Lung Diseases: From Molecules to Clinical Implications.Int J Mol Sci. 2019 Jun 19;20(12):2996. doi: 10.3390/ijms20122996. Int J Mol Sci. 2019. PMID: 31248154 Free PMC article. Review.
References
-
- Praveen K, Wen Y, Gray KM, Noto JJ, Patlolla AR, Van Duyne GD, Matera AG. SMA-causing missense mutations in survival motor neuron (Smn) display a wide range of phenotypes when modeled in Drosophila. PLoS Genet 2014; 10:e1004489; PMID:25144193; https://doi.org/10.1371/journal.pgen.1004489 - DOI - PMC - PubMed
-
- Kiss T. Biogenesis of small nuclear RNPs. J Cell Sci 2004; 117:5949-51; PMID:15564372; https://doi.org/10.1242/jcs.01487 - DOI - PubMed
-
- Egan ED, Collins K. Biogenesis of telomerase ribonucleoproteins. Rna 2012; 18:1747-59; PMID:22875809; https://doi.org/10.1261/rna.034629.112 - DOI - PMC - PubMed
-
- Trahan C, Dragon F. Dyskeratosis congenita mutations in the H/ACA domain of human telomerase RNA affect its assembly into a pre-RNP. Rna 2009; 15:235-43; PMID:19095616; https://doi.org/10.1261/rna.1354009 - DOI - PMC - PubMed
-
- Raska I, Andrade LEC, Ochs RL, Chan EKL, Chang C-M, Roos G, Tan EM. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res 1991; 195:27-37; PMID:2055273; https://doi.org/10.1016/0014-4827(91)90496-H - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
