Trapping redox partnerships in oxidant-sensitive proteins with a small, thiol-reactive cross-linker
- PMID: 27816612
- PMCID: PMC5154803
- DOI: 10.1016/j.freeradbiomed.2016.10.506
Trapping redox partnerships in oxidant-sensitive proteins with a small, thiol-reactive cross-linker
Abstract
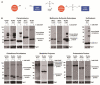
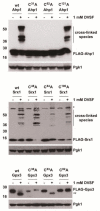
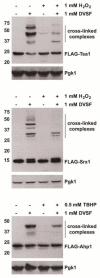
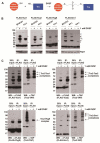
A broad range of redox-regulated proteins undergo reversible disulfide bond formation on oxidation-prone cysteine residues. Heightened reactivity of the thiol groups in these cysteines also increases susceptibility to modification by organic electrophiles, a property that can be exploited in the study of redox networks. Here, we explored whether divinyl sulfone (DVSF), a thiol-reactive bifunctional electrophile, cross-links oxidant-sensitive proteins to their putative redox partners in cells. To test this idea, previously identified oxidant targets involved in oxidant defense (namely, peroxiredoxins, methionine sulfoxide reductases, sulfiredoxin, and glutathione peroxidases), metabolism, and proteostasis were monitored for cross-link formation following treatment of Saccharomyces cerevisiae with DVSF. Several proteins screened, including multiple oxidant defense proteins, underwent intermolecular and/or intramolecular cross-linking in response to DVSF. Specific redox-active cysteines within a subset of DVSF targets were found to influence cross-linking; in addition, DVSF-mediated cross-linking of its targets was impaired in cells first exposed to oxidants. Since cross-linking appeared to involve redox-active cysteines in these proteins, we examined whether potential redox partners became cross-linked to them upon DVSF treatment. Specifically, we found that several substrates of thioredoxins were cross-linked to the cytosolic thioredoxin Trx2 in cells treated with DVSF. However, other DVSF targets, like the peroxiredoxin Ahp1, principally formed intra-protein cross-links upon DVSF treatment. Moreover, additional protein targets, including several known to undergo S-glutathionylation, were conjugated via DVSF to glutathione. Our results indicate that DVSF is of potential use as a chemical tool for irreversibly trapping and discovering thiol-based redox partnerships within cells.
Keywords: Cross-linker; Disulfide; Electrophile; Glutathione peroxidase; Glutathionylation; Methionine sulfoxide reductase; Peroxiredoxin; Sulfiredoxin; Thiol; Thioredoxin.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Identifying Interaction Partners of Yeast Protein Disulfide Isomerases Using a Small Thiol-Reactive Cross-Linker: Implications for Secretory Pathway Proteostasis.Chem Res Toxicol. 2022 Feb 21;35(2):326-336. doi: 10.1021/acs.chemrestox.1c00376. Epub 2022 Jan 27. Chem Res Toxicol. 2022. PMID: 35084835 Free PMC article.
-
Bifunctional electrophiles cross-link thioredoxins with redox relay partners in cells.Chem Res Toxicol. 2013 Mar 18;26(3):490-7. doi: 10.1021/tx4000123. Epub 2013 Mar 4. Chem Res Toxicol. 2013. PMID: 23414292 Free PMC article.
-
A crosslinker-based identification of redox relay targets.Anal Biochem. 2017 Mar 1;520:22-26. doi: 10.1016/j.ab.2016.12.025. Epub 2016 Dec 31. Anal Biochem. 2017. PMID: 28048978
-
Measurement and meaning of cellular thiol:disufhide redox status.Free Radic Res. 2016;50(2):246-71. doi: 10.3109/10715762.2015.1110241. Free Radic Res. 2016. PMID: 26695718 Review.
-
Chemistry and Enzymology of Disulfide Cross-Linking in Proteins.Chem Rev. 2018 Feb 14;118(3):1169-1198. doi: 10.1021/acs.chemrev.7b00123. Epub 2017 Jul 12. Chem Rev. 2018. PMID: 28699750 Free PMC article. Review.
Cited by
-
Aromatic Residues at the Dimer-Dimer Interface in the Peroxiredoxin Tsa1 Facilitate Decamer Formation and Biological Function.Chem Res Toxicol. 2019 Mar 18;32(3):474-483. doi: 10.1021/acs.chemrestox.8b00346. Epub 2019 Feb 11. Chem Res Toxicol. 2019. PMID: 30701970 Free PMC article.
-
Activity-based Crosslinking to Identify Substrates of Thioredoxin-domain Proteinsin Malaria Parasites.Bio Protoc. 2022 Feb 20;12(4):e4322. doi: 10.21769/BioProtoc.4322. eCollection 2022 Feb 20. Bio Protoc. 2022. PMID: 35340291 Free PMC article.
-
Piecing Together How Peroxiredoxins Maintain Genomic Stability.Antioxidants (Basel). 2018 Nov 28;7(12):177. doi: 10.3390/antiox7120177. Antioxidants (Basel). 2018. PMID: 30486489 Free PMC article. Review.
-
Identifying Interaction Partners of Yeast Protein Disulfide Isomerases Using a Small Thiol-Reactive Cross-Linker: Implications for Secretory Pathway Proteostasis.Chem Res Toxicol. 2022 Feb 21;35(2):326-336. doi: 10.1021/acs.chemrestox.1c00376. Epub 2022 Jan 27. Chem Res Toxicol. 2022. PMID: 35084835 Free PMC article.
-
Cytoplasmic redox imbalance in the thioredoxin system activates Hsf1 and results in hyperaccumulation of the sequestrase Hsp42 with misfolded proteins.Mol Biol Cell. 2024 Apr 1;35(4):ar53. doi: 10.1091/mbc.E23-07-0296. Epub 2024 Feb 21. Mol Biol Cell. 2024. PMID: 38381577 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

