Emerging concepts and future challenges in innate lymphoid cell biology
- PMID: 27811053
- PMCID: PMC5068238
- DOI: 10.1084/jem.20160525
Emerging concepts and future challenges in innate lymphoid cell biology
Abstract
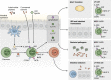
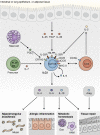
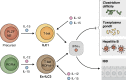
Innate lymphoid cells (ILCs) are innate immune cells that are ubiquitously distributed in lymphoid and nonlymphoid tissues and enriched at mucosal and barrier surfaces. Three major ILC subsets are recognized in mice and humans. Each of these subsets interacts with innate and adaptive immune cells and integrates cues from the epithelium, the microbiota, and pathogens to regulate inflammation, immunity, tissue repair, and metabolic homeostasis. Although intense study has elucidated many aspects of ILC development, phenotype, and function, numerous challenges remain in the field of ILC biology. In particular, recent work has highlighted key new questions regarding how these cells communicate with their environment and other cell types during health and disease. This review summarizes new findings in this rapidly developing field that showcase the critical role ILCs play in directing immune responses through their ability to interact with a variety of hematopoietic and nonhematopoietic cells. In addition, we define remaining challenges and emerging questions facing the field. Finally, this review discusses the potential application of basic studies of ILC biology to the development of new treatments for human patients with inflammatory and infectious diseases in which ILCs play a role.
© 2016 Tait Wojno and Artis.
Figures
Similar articles
-
Innate lymphoid cells, possible interaction with microbiota.Semin Immunopathol. 2015 Jan;37(1):27-37. doi: 10.1007/s00281-014-0470-4. Epub 2014 Dec 13. Semin Immunopathol. 2015. PMID: 25502370 Free PMC article. Review.
-
Dichotomous Regulation of Acquired Immunity by Innate Lymphoid Cells.Cells. 2020 May 11;9(5):1193. doi: 10.3390/cells9051193. Cells. 2020. PMID: 32403291 Free PMC article. Review.
-
Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis.Nat Immunol. 2016 Jun 21;17(7):765-74. doi: 10.1038/ni.3489. Nat Immunol. 2016. PMID: 27328006 Review.
-
Chemokine regulation of innate lymphoid cell tissue distribution and function.Cytokine Growth Factor Rev. 2018 Aug;42:47-55. doi: 10.1016/j.cytogfr.2018.02.003. Epub 2018 Feb 14. Cytokine Growth Factor Rev. 2018. PMID: 29472011 Review.
-
Helper-like Innate Lymphoid Cells in Humans and Mice.Trends Immunol. 2020 May;41(5):436-452. doi: 10.1016/j.it.2020.03.002. Epub 2020 Mar 26. Trends Immunol. 2020. PMID: 32223931 Review.
Cited by
-
Clostridioides difficile Toxin B Activates Group 3 Innate Lymphocytes.Infect Immun. 2022 Apr 21;90(4):e0007322. doi: 10.1128/iai.00073-22. Epub 2022 Apr 4. Infect Immun. 2022. PMID: 35377172 Free PMC article.
-
The Znt7-null mutation has sex dependent effects on the gut microbiota and goblet cell population in the mouse colon.PLoS One. 2020 Sep 29;15(9):e0239681. doi: 10.1371/journal.pone.0239681. eCollection 2020. PLoS One. 2020. PMID: 32991615 Free PMC article.
-
Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies.Front Immunol. 2020 Sep 4;11:2054. doi: 10.3389/fimmu.2020.02054. eCollection 2020. Front Immunol. 2020. PMID: 33013869 Free PMC article. Review.
-
Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress ILC2-Dependent Airway Inflammation.Front Immunol. 2019 Sep 18;10:2051. doi: 10.3389/fimmu.2019.02051. eCollection 2019. Front Immunol. 2019. PMID: 31620118 Free PMC article.
-
Suppression of ILC2 differentiation from committed T cell precursors by E protein transcription factors.J Exp Med. 2019 Apr 1;216(4):884-899. doi: 10.1084/jem.20182100. Epub 2019 Mar 21. J Exp Med. 2019. PMID: 30898894 Free PMC article.
References
-
- Abt M.C., Lewis B.B., Caballero S., Xiong H., Carter R.A., Sušac B., Ling L., Leiner I., and Pamer E.G.. 2015. Innate immune defenses Mediated by two ILC subsets are critical for protection against acute Clostridium difficile infection. Cell Host Microbe. 18:27–37. 10.1016/j.chom.2015.06.011 - DOI - PMC - PubMed
-
- Abt M.C., Buffie C.G., Sušac B., Becattini S., Carter R.A., Leiner I., Keith J.W., Artis D., Osborne L.C., and Pamer E.G.. 2016. TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant enterococcus. Sci. Transl. Med. 8:327ra25 10.1126/scitranslmed.aad6663 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

