Heterologous Immunity and Persistent Murine Cytomegalovirus Infection
- PMID: 27807227
- PMCID: PMC5215326
- DOI: 10.1128/JVI.01386-16
Heterologous Immunity and Persistent Murine Cytomegalovirus Infection
Abstract
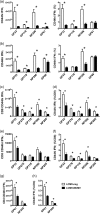
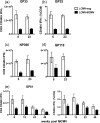
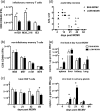
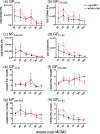
One's history of infections can affect the immune response to unrelated pathogens and influence disease outcome through the process of heterologous immunity. This can occur after acute viral infections, such as infections with lymphocytic choriomeningitis virus (LCMV) and vaccinia virus, where the pathogens are cleared, but it becomes a more complex issue in the context of persistent infections. In this study, murine cytomegalovirus (MCMV) was used as a persistent infection model to study heterologous immunity with LCMV. If mice were previously immune to LCMV and then infected with MCMV (LCMV+MCMV), they had more severe immunopathology, enhanced viral burden in multiple organs, and suppression of MCMV-specific T cell memory inflation. MCMV infection initially reduced the numbers of LCMV-specific memory T cells, but continued MCMV persistence did not further erode memory T cells specific to LCMV. When MCMV infection was given first (MCMV+LCMV), the magnitude of the acute T cell response to LCMV declined with age though this age-dependent decline was not dependent on MCMV. However, some of these MCMV persistently infected mice with acute LCMV infection (7 of 36) developed a robust immunodominant CD8 T cell response apparently cross-reactive between a newly defined putative MCMV epitope sequence, M57727-734, and the normally subdominant LCMV epitope L2062-2069, indicating a profound private specificity effect in heterologous immunity between these two viruses. These results further illustrate how a history of an acute or a persistent virus infection can substantially influence the immune responses and immune pathology associated with acute or persistent infections with an unrelated virus.
Importance: This study extends our understanding of heterologous immunity in the context of persistent viral infection. The phenomenon has been studied mostly with viruses such as LCMV that are cleared, but the situation can be more complex with a persistent virus such as MCMV. We found that the history of LCMV infection intensifies MCMV immunopathology, enhances MCMV burden in multiple organs, and suppresses MCMV-specific T cell memory inflation. In the reverse infection sequence, we show that some of the long-term MCMV-immune mice mount a robust CD8 T cell cross-reactive response between a newly defined putative MCMV epitope sequence and a normally subdominant LCMV epitope. These results further illustrate how a history of infection can substantially influence the immune responses and immune pathology associated with infections with an unrelated virus.
Keywords: CD8 T cell; cross-reactivity; heterologous immunity; lymphocytic choriomeningitis virus; memory inflation; mouse; murine cytomegalovirus.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections.J Immunol. 2003 Apr 15;170(8):4077-86. doi: 10.4049/jimmunol.170.8.4077. J Immunol. 2003. PMID: 12682237
-
Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections.J Exp Med. 1996 Jun 1;183(6):2489-99. doi: 10.1084/jem.183.6.2489. J Exp Med. 1996. PMID: 8676069 Free PMC article.
-
Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses.J Exp Med. 1994 Jun 1;179(6):1933-43. doi: 10.1084/jem.179.6.1933. J Exp Med. 1994. PMID: 8195718 Free PMC article.
-
Viral escape mechanisms--escapology taught by viruses.Int J Exp Pathol. 2001 Oct;82(5):269-86. doi: 10.1046/j.1365-2613.2001.00204.x. Int J Exp Pathol. 2001. PMID: 11703537 Free PMC article. Review.
-
Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future.Viruses. 2012 Oct 29;4(11):2650-69. doi: 10.3390/v4112650. Viruses. 2012. PMID: 23202498 Free PMC article. Review.
Cited by
-
Features of Effective T Cell-Inducing Vaccines against Chronic Viral Infections.Front Immunol. 2018 Feb 16;9:276. doi: 10.3389/fimmu.2018.00276. eCollection 2018. Front Immunol. 2018. PMID: 29503649 Free PMC article. Review.
-
A constant companion: immune recognition and response to cytomegalovirus with aging and implications for immune fitness.Geroscience. 2017 Jun;39(3):293-303. doi: 10.1007/s11357-017-9982-x. Epub 2017 Jun 24. Geroscience. 2017. PMID: 28647907 Free PMC article. Review.
-
The effect of respiratory viruses on immunogenicity and protection induced by a candidate universal influenza vaccine in mice.PLoS One. 2019 Apr 15;14(4):e0215321. doi: 10.1371/journal.pone.0215321. eCollection 2019. PLoS One. 2019. PMID: 30986224 Free PMC article.
-
Latent Cytomegalovirus Infection in Female Mice Increases Breast Cancer Metastasis.Cancers (Basel). 2019 Mar 29;11(4):447. doi: 10.3390/cancers11040447. Cancers (Basel). 2019. PMID: 30934926 Free PMC article.
-
Virological and Immunological Outcomes of Coinfections.Clin Microbiol Rev. 2018 Jul 5;31(4):e00111-17. doi: 10.1128/CMR.00111-17. Print 2018 Oct. Clin Microbiol Rev. 2018. PMID: 29976554 Free PMC article. Review.
References
-
- Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40:444–451. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

