MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients
- PMID: 27802824
- PMCID: PMC5088645
- DOI: 10.1186/s12974-016-0720-6
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients
Abstract
Background: Antibodies against myelin oligodendrocyte glycoprotein (MOG-IgG) have been reported in patients with aquaporin-4 antibody (AQP4-IgG)-negative neuromyelitis optica spectrum disorders (NMOSD). The objective of this study was to describe optic neuritis (ON)-induced neuro-axonal damage in the retina of MOG-IgG-positive patients in comparison with AQP4-IgG-positive NMOSD patients.
Methods: Afferent visual system damage following ON was bilaterally assessed in 16 MOG-IgG-positive patients with a history of ON and compared with that in 16 AQP4-IgG-positive NMOSD patients. In addition, 16 healthy controls matched for age, sex, and disease duration were analyzed. Study data included ON history, retinal optical coherence tomography, visual acuity, and visual evoked potentials.
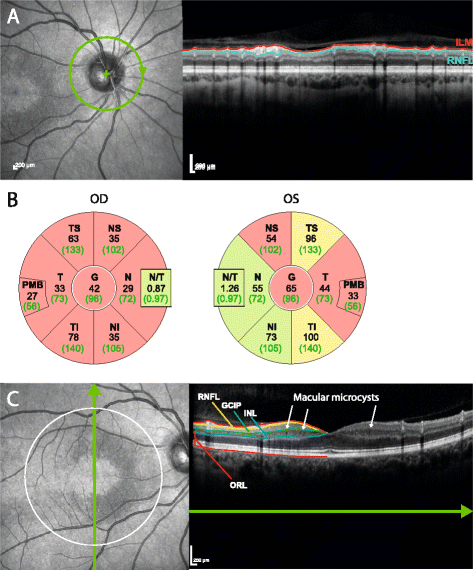
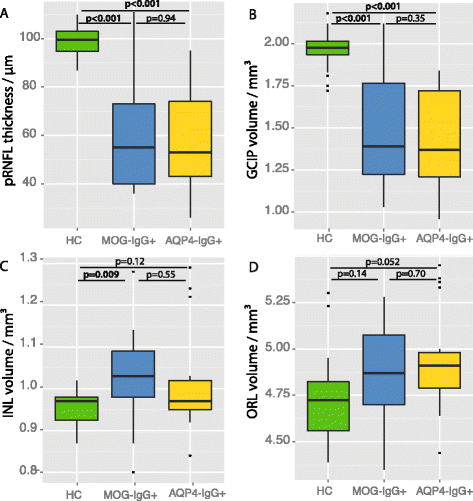
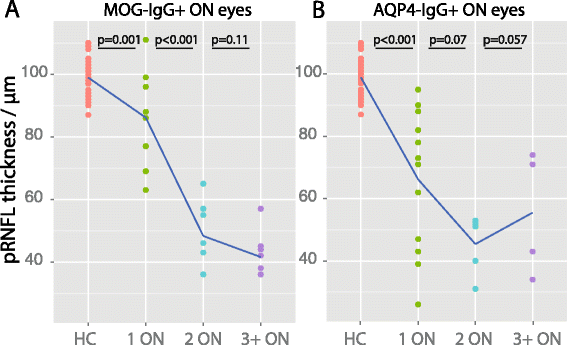
Results: Eight MOG-IgG-positive patients had a previous diagnosis of AQP4-IgG-negative NMOSD with ON and myelitis, and eight of (mainly recurrent) ON. Twenty-nine of the 32 eyes of the MOG-IgG-positive patients had been affected by at least one episode of ON. Peripapillary retinal nerve fiber layer thickness (pRNFL) and ganglion cell and inner plexiform layer volume (GCIP) were significantly reduced in ON eyes of MOG-IgG-positive patients (pRNFL = 59 ± 23 μm; GCIP = 1.50 ± 0.34 mm3) compared with healthy controls (pRNFL = 99 ± 6 μm, p < 0.001; GCIP = 1.97 ± 0.11 mm3, p < 0.001). Visual acuity was impaired in eyes after ON in MOG-IgG-positive patients (0.35 ± 0.88 logMAR). There were no significant differences in any structural or functional visual parameters between MOG-IgG-positive and AQP4-IgG-positive patients (pRNFL: 59 ± 21 μm; GCIP: 1.41 ± 0.27 mm3; Visual acuity = 0.72 ± 1.09 logMAR). Importantly, MOG-IgG-positive patients had a significantly higher annual ON relapse rate than AQP4-IgG-positive patients (median 0.69 vs. 0.29 attacks/year, p = 0.004), meaning that on average a single ON episode caused less damage in MOG-IgG-positive than in AQP4-IgG-positive patients. pRNFL and GCIP loss correlated with the number of ON episodes in MOG-IgG-positive patients (p < 0.001), but not in AQP4-IgG-positive patients.
Conclusions: Retinal neuro-axonal damage and visual impairment after ON in MOG-IgG-positive patients are as severe as in AQP4-IgG-positive NMOSD patients. In MOG-IgG-positive patients, damage accrual may be driven by higher relapse rates, whereas AQP4-IgG-positive patients showed fewer but more severe episodes of ON. Given the marked damage in some of our MOG-IgG-positive patients, early diagnosis and timely initiation and close monitoring of immunosuppressive therapy are important.
Keywords: Devic syndrome; Myelin oligodendrocyte glycoprotein antibodies (MOG-IgG); NMO-IgG; aquaporin-4 antibodies (AQP4-IgG); neuromyelitis optica; neuromyelitis optica spectrum disorders (NMOSD); optic neuritis; optical coherence tomography; retinal neuro-axonal damage; visual acuity; visual evoked potentials.
Figures
Similar articles
-
Neuromyelitis optica spectrum disorders with antibodies to myelin oligodendrocyte glycoprotein or aquaporin-4: Clinical and paraclinical characteristics in Algerian patients.J Neurol Sci. 2017 Oct 15;381:240-244. doi: 10.1016/j.jns.2017.08.3254. Epub 2017 Aug 31. J Neurol Sci. 2017. PMID: 28991690
-
Optical coherence tomography in myelin-oligodendrocyte-glycoprotein antibody-seropositive patients: a longitudinal study.J Neuroinflammation. 2019 Jul 25;16(1):154. doi: 10.1186/s12974-019-1521-5. J Neuroinflammation. 2019. PMID: 31345223 Free PMC article.
-
Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration.J Neurol. 2017 Jan;264(1):139-151. doi: 10.1007/s00415-016-8333-7. Epub 2016 Nov 14. J Neurol. 2017. PMID: 27844165
-
Retina thickness in clinically affected and unaffected eyes in patients with aquaporin-4 immunoglobulin G antibody seropositive neuromyelitis optica spectrum disorders: a systematic review and meta-analysis.J Neurol. 2023 Feb;270(2):759-768. doi: 10.1007/s00415-022-11482-4. Epub 2022 Nov 10. J Neurol. 2023. PMID: 36355186 Review.
-
Changes of retinal structure and visual function in patients with demyelinating transverse myelitis.Neurol Sci. 2022 Nov;43(11):6425-6431. doi: 10.1007/s10072-022-06315-1. Epub 2022 Aug 8. Neurol Sci. 2022. PMID: 35939134 Review.
Cited by
-
MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin.J Neuroinflammation. 2016 Sep 26;13(1):279. doi: 10.1186/s12974-016-0717-1. J Neuroinflammation. 2016. PMID: 27788675 Free PMC article.
-
The neuro-ophthalmological manifestations of NMOSD and MOGAD-a comprehensive review.Eye (Lond). 2023 Aug;37(12):2391-2398. doi: 10.1038/s41433-023-02477-0. Epub 2023 Mar 16. Eye (Lond). 2023. PMID: 36928226 Free PMC article. Review.
-
Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome.Am J Ophthalmol. 2018 Nov;195:8-15. doi: 10.1016/j.ajo.2018.07.020. Epub 2018 Jul 26. Am J Ophthalmol. 2018. PMID: 30055153 Free PMC article.
-
MOG-IgG-Associated Optic Neuritis, Encephalitis, and Myelitis: Lessons Learned From Neuromyelitis Optica Spectrum Disorder.Front Neurol. 2018 Apr 4;9:217. doi: 10.3389/fneur.2018.00217. eCollection 2018. Front Neurol. 2018. PMID: 29670575 Free PMC article. Review.
-
The Detection of Retina Microvascular Density in Subclinical Aquaporin-4 Antibody Seropositive Neuromyelitis Optica Spectrum Disorders.Front Neurol. 2020 Feb 11;11:35. doi: 10.3389/fneur.2020.00035. eCollection 2020. Front Neurol. 2020. PMID: 32117008 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

