Administration of Menadione, Vitamin K3, Ameliorates Off-Target Effects on Corneal Epithelial Wound Healing Due to Receptor Tyrosine Kinase Inhibition
- PMID: 27802516
- PMCID: PMC6733502
- DOI: 10.1167/iovs.16-19952
Administration of Menadione, Vitamin K3, Ameliorates Off-Target Effects on Corneal Epithelial Wound Healing Due to Receptor Tyrosine Kinase Inhibition
Abstract
Purpose: The antiangiogenic receptor tyrosine kinase inhibitor (RTKi), 3-[(4-bromo-2,6-difluorophenyl)methoxy]-5-[[[[4-(1-pyrrolidinyl) butyl] amino] carbonyl]amino]-4-isothiazolecarboxamide hydrochloride, targets VEGFR2 (half maximal inhibitory concentration [IC50] = 11 nM); however, off-target inhibition of epidermal growth factor receptor (EGFR) occurs at higher concentrations. (IC50 = 5.8 μM). This study was designed to determine the effect of topical RTKi treatment on EGF-mediated corneal epithelial wound healing and to develop new strategies to minimize off-target EGFR inhibition.
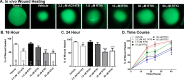
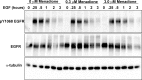
Methods: In vitro corneal epithelial wound healing was measured in response to EGF using a transformed human cell line (hTCEpi cells). In vivo corneal wound healing was assessed using a murine model. In these complementary assays, wound healing was measured in the presence of varying RTKi concentrations. Immunoblot analysis was used to examine EGFR and VEGFR2 phosphorylation and the kinetics of EGFR degradation. An Alamar Blue assay measured VEGFR2-mediated cell biology.
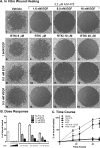
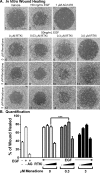
Results: Receptor tyrosine kinase inhibitor exposure caused dose-dependent inhibition of EGFR-mediated corneal epithelial wound healing in vitro and in vivo. Nanomolar concentrations of menadione, a vitamin K3 analog, when coadministered with the RTKi, slowed EGFR degradation and ameliorated the inhibitory effects on epithelial wound healing both in vitro and in vivo. Menadione did not alter the RTKi's IC50 against VEGFR2 phosphorylation or its inhibition of VEGF-induced retinal endothelial cell proliferation.
Conclusions: An antiangiogenic RTKi exhibited off-target effects on the corneal epithelium that can be minimized by menadione without deleteriously affecting its on-target VEGFR2 blockade. These data indicate that menadione has potential as a topical supplement for individuals suffering from perturbations in corneal epithelial homeostasis, especially as an untoward side effect of kinase inhibitors.
Figures

Similar articles
-
Antagonizing c-Cbl enhances EGFR-dependent corneal epithelial homeostasis.Invest Ophthalmol Vis Sci. 2014 Jul 1;55(8):4691-9. doi: 10.1167/iovs.14-14133. Invest Ophthalmol Vis Sci. 2014. PMID: 24985478 Free PMC article.
-
Lidocaine inhibits tyrosine kinase activity of the epidermal growth factor receptor and suppresses proliferation of corneal epithelial cells.Anesthesiology. 2004 May;100(5):1206-10. doi: 10.1097/00000542-200405000-00024. Anesthesiology. 2004. PMID: 15114219
-
Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells.Invest Ophthalmol Vis Sci. 2004 Mar;45(3):813-20. doi: 10.1167/iovs.03-0851. Invest Ophthalmol Vis Sci. 2004. PMID: 14985295 Free PMC article.
-
Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor.Invest Ophthalmol Vis Sci. 2007 Feb;48(2):636-43. doi: 10.1167/iovs.06-0203. Invest Ophthalmol Vis Sci. 2007. PMID: 17251460 Free PMC article.
-
Epidermal Growth Factor Stimulates Transforming Growth Factor-Beta Receptor Type II Expression In Corneal Epithelial Cells.Sci Rep. 2019 May 30;9(1):8079. doi: 10.1038/s41598-019-42969-2. Sci Rep. 2019. PMID: 31147562 Free PMC article.
Cited by
-
Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration.J Transl Med. 2023 Feb 21;21(1):133. doi: 10.1186/s12967-023-03937-7. J Transl Med. 2023. PMID: 36810060 Free PMC article. Review.
-
Age-Related Macular Degeneration (AMD): Pathophysiology, Drug Targeting Approaches, and Recent Developments in Nanotherapeutics.Medicina (Kaunas). 2024 Oct 8;60(10):1647. doi: 10.3390/medicina60101647. Medicina (Kaunas). 2024. PMID: 39459435 Free PMC article. Review.
-
Epithelial Cell Migration and Proliferation Patterns During Initial Wound Closure in Normal Mice and an Experimental Model of Limbal Stem Cell Deficiency.Invest Ophthalmol Vis Sci. 2020 Aug 3;61(10):27. doi: 10.1167/iovs.61.10.27. Invest Ophthalmol Vis Sci. 2020. PMID: 32790859 Free PMC article.
-
Epidermal Growth Factor Receptor Expression in the Corneal Epithelium.Cells. 2021 Sep 13;10(9):2409. doi: 10.3390/cells10092409. Cells. 2021. PMID: 34572058 Free PMC article. Review.
-
Collagen Mimetic Peptides Promote Corneal Epithelial Cell Regeneration.Front Pharmacol. 2021 Aug 16;12:705623. doi: 10.3389/fphar.2021.705623. eCollection 2021. Front Pharmacol. 2021. PMID: 34483909 Free PMC article.
References
-
- Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. - PubMed
-
- Beebe JS, Jani JP, Knauth E, et al. Pharmacological characterization of CP-547,632, a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for cancer therapy. Cancer Res. 2003;63:7301–7309. - PubMed
-
- Cohen RB, Langer CJ, Simon GR, et al. A phase I/randomized phase II, non-comparative, multicenter open label trial of CP-547632 in combination with paclitaxel and carboplatin or paclitaxel and carboplatin alone as first-line treatment for advanced non-small cell lung cancer (NSCLC) Cancer Chemother Pharmacol. 2007;60:81–89. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

