Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis
- PMID: 27799946
- PMCID: PMC5084469
- DOI: 10.1186/s13633-016-0038-2
Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis
Abstract
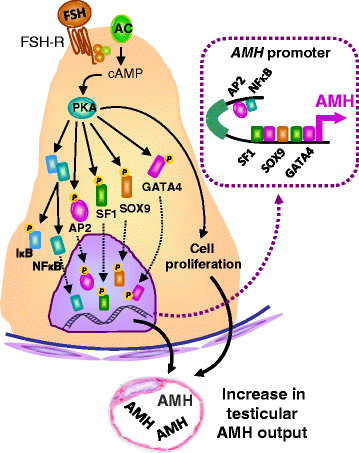
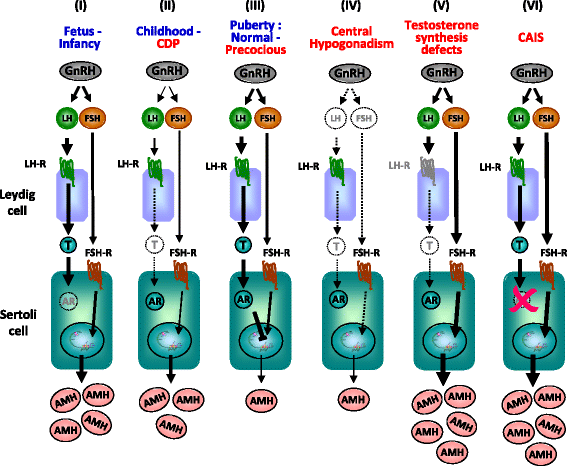
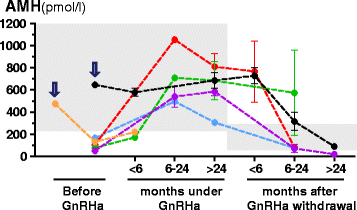
In pediatric patients, basal testosterone and gonadotropin levels may be uninformative in the assessment of testicular function. Measurement of serum anti-Müllerian hormone (AMH) has become increasingly widespread since it provides information about the activity of the male gonad without the need for dynamic tests, and also reflects the action of FSH and androgens within the testis. AMH is secreted in high amounts by Sertoli cells from fetal life until the onset of puberty. Basal AMH expression is not dependent on gonadotropins or sex steroids; however, FSH further increases and testosterone inhibits AMH production. During puberty, testosterone induces Sertoli cell maturation, and prevails over FSH on AMH regulation. Therefore, AMH production decreases. Serum AMH is undetectable in patients with congenital or acquired anorchidism, or with complete gonadal dysgenesis. Low circulating levels of AMH may reflect primary testicular dysfunction, e.g. in certain patients with cryptorchidism, monorchidism, partial gonadal dysgenesis, or central hypogonadism. AMH is low in boys with precocious puberty, but it increases to prepubertal levels after successful treatment. Conversely, serum AMH remains at high, prepubertal levels in boys with constitutional delay of puberty. Serum AMH measurements are useful, together with testosterone determination, in the diagnosis of patients with ambiguous genitalia: both are low in patients with gonadal dysgenesis, including ovotesticular disorders of sex development, testosterone is low but AMH is in the normal male range or higher in patients with disorders of androgen synthesis, and both hormones are normal or high in patients with androgen insensitivity. Finally, elevation of serum AMH above normal male prepubertal levels may be indicative of rare cases of sex-cord stromal tumors or Sertoli cell-limited disturbance in the McCune Albright syndrome.
Keywords: Cryptorchidism; Disorders of sex development; Puberty; Sertoli; Testis.
Figures

Similar articles
-
[Anti-Müllerian hormone (AMH) measurements in the assessment of testicular function in prepubertal boys and in sexual differentiation disorders].Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2006;12(3):195-9. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2006. PMID: 17020655 Polish.
-
Anti-Müllerian hormone, testicular descent and cryptorchidism.Front Endocrinol (Lausanne). 2024 Mar 4;15:1361032. doi: 10.3389/fendo.2024.1361032. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38501100 Free PMC article. Review.
-
Anti-müllerian hormone and sertoli cell function in paediatric male hypogonadism.Horm Res Paediatr. 2010;73(2):81-92. doi: 10.1159/000277140. Epub 2010 Feb 9. Horm Res Paediatr. 2010. PMID: 20190544 Review.
-
Anti-müllerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development.J Clin Endocrinol Metab. 1993 Nov;77(5):1220-6. doi: 10.1210/jcem.77.5.8077315. J Clin Endocrinol Metab. 1993. PMID: 8077315
-
New perspectives in the diagnosis of pediatric male hypogonadism: the importance of AMH as a Sertoli cell marker.Arq Bras Endocrinol Metabol. 2011 Nov;55(8):512-9. doi: 10.1590/s0004-27302011000800003. Arq Bras Endocrinol Metabol. 2011. PMID: 22218431 Review.
Cited by
-
Anti-Müllerian hormone: biology and role in endocrinology and cancers.Front Endocrinol (Lausanne). 2024 Sep 16;15:1468364. doi: 10.3389/fendo.2024.1468364. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39351532 Free PMC article. Review.
-
Evaluation of testicular volume in males with congenital hypogonadotropic hypogonadism: a comparative analysis.Endocrine. 2024 Sep 25. doi: 10.1007/s12020-024-04032-7. Online ahead of print. Endocrine. 2024. PMID: 39320590
-
Analytical performances of a novel fluorescent immunoassay of anti-Müllerian hormone and establishment of the reference intervals in Chinese children.Pract Lab Med. 2024 Jul 16;41:e00419. doi: 10.1016/j.plabm.2024.e00419. eCollection 2024 Aug. Pract Lab Med. 2024. PMID: 39205827 Free PMC article.
-
Outcomes and experiences of adults with congenital hypogonadism can inform improvements in the management of delayed puberty.J Pediatr Endocrinol Metab. 2023 Nov 24;37(1):1-7. doi: 10.1515/jpem-2023-0407. Print 2024 Jan 29. J Pediatr Endocrinol Metab. 2023. PMID: 37997801 Free PMC article. Review.
-
Biomarkers of male hypogonadism in childhood and adolescence.Adv Lab Med. 2020 Apr 21;1(2):20200024. doi: 10.1515/almed-2020-0024. eCollection 2020 Jun. Adv Lab Med. 2020. PMID: 37363780 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

