Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs
- PMID: 27798617
- PMCID: PMC7097164
- DOI: 10.1038/ni.3566
Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs
Abstract
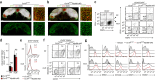
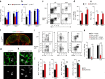
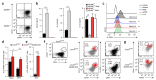
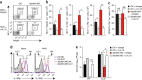
Fibroblastic reticular cells (FRCs) of secondary lymphoid organs form distinct niches for interaction with hematopoietic cells. We found here that production of the cytokine IL-15 by FRCs was essential for the maintenance of group 1 innate lymphoid cells (ILCs) in Peyer's patches and mesenteric lymph nodes. Moreover, FRC-specific ablation of the innate immunological sensing adaptor MyD88 unleashed IL-15 production by FRCs during infection with an enteropathogenic virus, which led to hyperactivation of group 1 ILCs and substantially altered the differentiation of helper T cells. Accelerated clearance of virus by group 1 ILCs precipitated severe intestinal inflammatory disease with commensal dysbiosis, loss of intestinal barrier function and diminished resistance to colonization. In sum, FRCs act as an 'on-demand' immunological 'rheostat' by restraining activation of group 1 ILCs and thereby preventing immunopathological damage in the intestine.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Fibroblastic reticular cell lineage convergence in Peyer's patches governs intestinal immunity.Nat Immunol. 2021 Apr;22(4):510-519. doi: 10.1038/s41590-021-00894-5. Epub 2021 Mar 11. Nat Immunol. 2021. PMID: 33707780 Free PMC article.
-
MyD88 signaling in dendritic cells and the intestinal epithelium controls immunity against intestinal infection with C. rodentium.PLoS Pathog. 2017 May 16;13(5):e1006357. doi: 10.1371/journal.ppat.1006357. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28520792 Free PMC article.
-
TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium.J Immunol. 2007 Jul 1;179(1):566-77. doi: 10.4049/jimmunol.179.1.566. J Immunol. 2007. PMID: 17579078
-
Fibroblastic reticular cells at the nexus of innate and adaptive immune responses.Immunol Rev. 2019 May;289(1):31-41. doi: 10.1111/imr.12748. Immunol Rev. 2019. PMID: 30977192 Free PMC article. Review.
-
Innate lymphoid cells in secondary lymphoid organs.Immunol Rev. 2016 May;271(1):185-99. doi: 10.1111/imr.12407. Immunol Rev. 2016. PMID: 27088915 Review.
Cited by
-
Insights into coronavirus immunity taught by the murine coronavirus.Eur J Immunol. 2021 May;51(5):1062-1070. doi: 10.1002/eji.202048984. Epub 2021 Mar 22. Eur J Immunol. 2021. PMID: 33687066 Free PMC article. Review.
-
The mesenchymal context in inflammation, immunity and cancer.Nat Immunol. 2020 Sep;21(9):974-982. doi: 10.1038/s41590-020-0741-2. Epub 2020 Aug 3. Nat Immunol. 2020. PMID: 32747813 Review.
-
Fibroblastic reticular cell lineage convergence in Peyer's patches governs intestinal immunity.Nat Immunol. 2021 Apr;22(4):510-519. doi: 10.1038/s41590-021-00894-5. Epub 2021 Mar 11. Nat Immunol. 2021. PMID: 33707780 Free PMC article.
-
Visualization and functional characterization of lymphoid organ fibroblasts.Immunol Rev. 2022 Mar;306(1):108-122. doi: 10.1111/imr.13051. Epub 2021 Dec 5. Immunol Rev. 2022. PMID: 34866192 Free PMC article. Review.
-
Fibroblastic reticular cells provide a supportive niche for lymph node-resident macrophages.Eur J Immunol. 2023 Sep;53(9):e2250355. doi: 10.1002/eji.202250355. Epub 2023 Jul 12. Eur J Immunol. 2023. PMID: 36991561 Free PMC article.
References
-
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. - PubMed
-
- Macpherson AJ, Hapfelmeier S, McCoy KD. The armed truce between the intestinal microflora and host mucosal immunity. Semin. Immunol. 2007;19:57–58. - PubMed
-
- Macpherson AJ, Geuking MB, Slack E, Hapfelmeier S, McCoy KD. The habitat, double life, citizenship, and forgetfulness of IgA. Immunol. Rev. 2012;245:132–146. - PubMed
-
- Huntington ND, Carpentier S, Vivier E, Belz GT. Innate lymphoid cells: parallel checkpoints and coordinate interactions with T cells. Curr. Opin. Immunol. 2016;38:86–93. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

