Transcriptional Silencing of Moloney Murine Leukemia Virus in Human Embryonic Carcinoma Cells
- PMID: 27795446
- PMCID: PMC5165191
- DOI: 10.1128/JVI.02075-16
Transcriptional Silencing of Moloney Murine Leukemia Virus in Human Embryonic Carcinoma Cells
Abstract
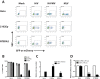
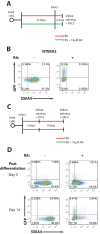
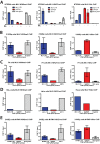
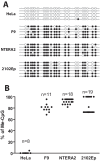
Embryonic carcinoma (EC) cells are malignant counterparts of embryonic stem (ES) cells and serve as useful models for investigating cellular differentiation and human embryogenesis. Though the susceptibility of murine EC cells to retroviral infection has been extensively analyzed, few studies of retrovirus infection of human EC cells have been performed. We tested the susceptibility of human EC cells to transduction by retroviral vectors derived from three different retroviral genera. We show that human EC cells efficiently express reporter genes delivered by vectors based on human immunodeficiency virus type 1 (HIV-1) and Mason-Pfizer monkey virus (M-PMV) but not Moloney murine leukemia virus (MLV). In human EC cells, MLV integration occurs normally, but no viral gene expression is observed. The block to MLV expression of MLV genomes is relieved upon cellular differentiation. The lack of gene expression is correlated with transcriptional silencing of the MLV promoter through the deposition of repressive histone marks as well as DNA methylation. Moreover, depletion of SETDB1, a histone methyltransferase, resulted in a loss of transcriptional silencing and upregulation of MLV gene expression. Finally, we provide evidence showing that the lack of MLV gene expression may be attributed in part to the lack of MLV enhancer function in human EC cells.
Importance: Human embryonic carcinoma (EC) cells are shown to restrict the expression of murine leukemia virus genomes but not retroviral genomes of the lentiviral or betaretroviral families. The block occurs at the level of transcription and is accompanied by the deposition of repressive histone marks and methylation of the integrated proviral DNA. The host machinery required for silencing in human EC cells is distinct from that in murine EC cell lines: the histone methyltransferase SETDB1 is required, but the widely utilized corepressor TRIM28/Kap1 is not. A transcriptional enhancer element from the Mason-Pfizer monkey virus can override the silencing and promote transcription of chimeric proviral DNAs. The findings reveal novel features of human EC gene regulation not present in their murine counterparts.
Keywords: DNA methylation; chromatin immunoprecipitation; enhancer; histones; repressor.
Copyright © 2016 American Society for Microbiology.
Figures

Similar articles
-
Ectopic DNMT3L triggers assembly of a repressive complex for retroviral silencing in somatic cells.J Virol. 2014 Sep;88(18):10680-95. doi: 10.1128/JVI.01176-14. Epub 2014 Jul 2. J Virol. 2014. PMID: 24991018 Free PMC article.
-
TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells.Cell. 2007 Oct 5;131(1):46-57. doi: 10.1016/j.cell.2007.07.026. Cell. 2007. PMID: 17923087
-
The Moloney murine leukemia virus repressor binding site represses expression in murine and human hematopoietic stem cells.J Virol. 2003 Sep;77(17):9439-50. doi: 10.1128/jvi.77.17.9439-9450.2003. J Virol. 2003. PMID: 12915559 Free PMC article.
-
Mechanisms that regulate silencing of gene expression from retroviral vectors.J Hematother Stem Cell Res. 2002 Jun;11(3):449-56. doi: 10.1089/15258160260090915. J Hematother Stem Cell Res. 2002. PMID: 12183830 Review.
-
Characterization of gP85gag as an antigen recognized by Moloney leukemia virus-specific cytolytic T cell clones that function in vivo.J Exp Med. 1985 Jul 1;162(1):128-44. doi: 10.1084/jem.162.1.128. J Exp Med. 1985. PMID: 3891902 Free PMC article. Review.
Cited by
-
Genome-wide CRISPR knockout screen identifies ZNF304 as a silencer of HIV transcription that promotes viral latency.PLoS Pathog. 2020 Sep 21;16(9):e1008834. doi: 10.1371/journal.ppat.1008834. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32956422 Free PMC article.
-
Epigenetic Silencing of Recombinant Adeno-associated Virus Genomes by NP220 and the HUSH Complex.J Virol. 2022 Feb 23;96(4):e0203921. doi: 10.1128/JVI.02039-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878926 Free PMC article.
-
CpG Methylation Profiles of HIV-1 Pro-Viral DNA in Individuals on ART.Viruses. 2021 Apr 29;13(5):799. doi: 10.3390/v13050799. Viruses. 2021. PMID: 33946976 Free PMC article.
-
Primate immunodeficiency virus proteins Vpx and Vpr counteract transcriptional repression of proviruses by the HUSH complex.Nat Microbiol. 2018 Dec;3(12):1354-1361. doi: 10.1038/s41564-018-0256-x. Epub 2018 Oct 8. Nat Microbiol. 2018. PMID: 30297740 Free PMC article.
References
-
- Andrews PW, Damjanov I, Simon D, Banting GS, Carlin C, Dracopoli NC, Fogh J. 1984. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest 50:147–162. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

