Importance of Highly Conserved Peptide Sites of Human Cytomegalovirus gO for Formation of the gH/gL/gO Complex
- PMID: 27795411
- PMCID: PMC5165187
- DOI: 10.1128/JVI.01339-16
Importance of Highly Conserved Peptide Sites of Human Cytomegalovirus gO for Formation of the gH/gL/gO Complex
Abstract
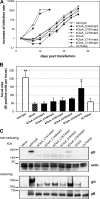
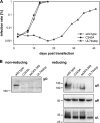
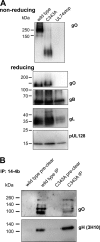
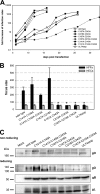
The glycoprotein O (gO) is betaherpesvirus specific. Together with the viral glycoproteins H and L, gO forms a covalent trimeric complex that is part of the viral envelope. This trimer is crucial for cell-free infectivity of human cytomegalovirus (HCMV) but dispensable for cell-associated spread. We hypothesized that the amino acids that are conserved among gOs of different cytomegaloviruses are important for the formation of the trimeric complex and hence for efficient virus spread. In a mutational approach, nine peptide sites, containing all 13 highly conserved amino acids, were analyzed in the context of HCMV strain TB40-BAC4 with regard to infection efficiency and formation of the gH/gL/gO complex. Mutation of amino acids (aa) 181 to 186 or aa 193 to 198 resulted in the loss of the trimer and a complete small-plaque phenotype, whereas mutation of aa 108 or aa 249 to 254 caused an intermediate phenotype. While individual mutations of the five conserved cysteines had little impact, their relevance was revealed in a combined mutation, which abrogated both complex formation and cell-free infectivity. C343 was unique, as it was sufficient and necessary for covalent binding of gO to gH/gL. Remarkably, however, C218 together with C167 rescued infectivity in the absence of detectable covalent complex formation. We conclude that all highly conserved amino acids contribute to the function of gO to some extent but that aa 181 to 198 and cysteines 343, 218, and 167 are particularly relevant. Surprisingly, covalent binding of gO to gH/gL is required neither for its incorporation into virions nor for proper function in cell-free infection.
Importance: Like all herpesviruses, the widespread human pathogen HCMV depends on glycoproteins gB, gH, and gL for entry into target cells. Additionally, gH and gL have to bind gO in a trimeric complex for efficient cell-free infection. Homologs of gO are shared by all cytomegaloviruses, with 13 amino acids being highly conserved. In a mutational approach we analyzed these amino acids to elucidate their role in the function of gO. All conserved amino acids contributed either to formation of the trimeric complex or to cell-free infection. Notably, these two phenotypes were not inevitably linked as the mutation of a charged cluster in the center of gO abrogated cell-free infection while trimeric complexes were still being formed. Cysteine 343 was essential for covalent binding of gO to gH/gL; however, noncovalent complex formation in the absence of cysteine 343 also allowed for cell-free infectivity.
Keywords: cell tropism; cytomegalovirus; glycoprotein O; glycoproteins; mutational studies.
Copyright © 2016 American Society for Microbiology.
Figures




Similar articles
-
Influence of Human Cytomegalovirus Glycoprotein O Polymorphism on the Inhibitory Effect of Soluble Forms of Trimer- and Pentamer-Specific Entry Receptors.J Virol. 2020 Jul 1;94(14):e00107-20. doi: 10.1128/JVI.00107-20. Print 2020 Jul 1. J Virol. 2020. PMID: 32350071 Free PMC article.
-
A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells.J Virol. 2010 Mar;84(5):2585-96. doi: 10.1128/JVI.02249-09. Epub 2009 Dec 23. J Virol. 2010. PMID: 20032184 Free PMC article.
-
The N Terminus of Human Cytomegalovirus Glycoprotein O Is Important for Binding to the Cellular Receptor PDGFRα.J Virol. 2019 May 15;93(11):e00138-19. doi: 10.1128/JVI.00138-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894468 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
-
Identification of functionally important domains of human cytomegalovirus gO that act after trimer binding to receptors.PLoS Pathog. 2022 Apr 22;18(4):e1010452. doi: 10.1371/journal.ppat.1010452. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35452493 Free PMC article.
-
Role of Envelope Glycoprotein Complexes in Cell-Associated Spread of Human Cytomegalovirus.Viruses. 2021 Apr 2;13(4):614. doi: 10.3390/v13040614. Viruses. 2021. PMID: 33918406 Free PMC article.
-
Natural Inhibitor of Human Cytomegalovirus in Human Seminal Plasma.J Virol. 2019 Mar 5;93(6):e01855-18. doi: 10.1128/JVI.01855-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626669 Free PMC article.
-
Differences in Growth Properties among Two Human Cytomegalovirus Glycoprotein O Genotypes.Front Microbiol. 2017 Aug 22;8:1609. doi: 10.3389/fmicb.2017.01609. eCollection 2017. Front Microbiol. 2017. PMID: 28878758 Free PMC article.
-
Protection against Congenital CMV Infection Conferred by MVA-Vectored Subunit Vaccines Extends to a Second Pregnancy after Maternal Challenge with a Heterologous, Novel Strain Variant.Viruses. 2021 Dec 20;13(12):2551. doi: 10.3390/v13122551. Viruses. 2021. PMID: 34960820 Free PMC article.
References
-
- Pass RF. 1996. Immunization strategy for prevention of congenital cytomegalovirus infection. Infect Agents Dis 5:240–244. - PubMed
-
- Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, Kuhr CS, Levy AE, Perkins JD, Reyes JD, Boeckh M. 2006. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 81:1645–1652. doi:10.1097/01.tp.0000226071.12562.1a. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

