Distinct HIV-1 Neutralization Potency Profiles of Ibalizumab-Based Bispecific Antibodies
- PMID: 27792681
- PMCID: PMC5123706
- DOI: 10.1097/QAI.0000000000001119
Distinct HIV-1 Neutralization Potency Profiles of Ibalizumab-Based Bispecific Antibodies
Abstract
Background: Preexposure prophylaxis using antiretroviral agents has been shown to effectively prevent human immunodeficiency virus type 1 (HIV-1) acquisition in high-risk populations. However, the efficacy of these regimens is highly variable, which is thought to be largely due to the varying degrees of adherence to a daily intervention in the populations. Passive immunization using broadly neutralizing antibodies (bNAbs) against HIV-1, with their relatively long half-life and favorable safety profile, could provide an alternative to daily preexposure prophylaxis. However, most bNAbs have a limited breadth, only neutralizing 70%-90% of all HIV-1 strains.
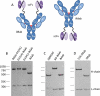
Methods: To overcome the problem of limited antiviral breadth, we proposed that targeting human CD4 and HIV-1 envelope proteins simultaneously may improve virus-neutralization breadth and potency. Therefore, we constructed bispecific antibodies (biAbs) using single-chain variable fragments of anti-gp120 bNAbs fused to ibalizumab (iMab), a humanized monoclonal antibody that binds human CD4, the primary receptor for HIV-1.
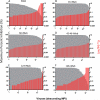
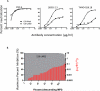
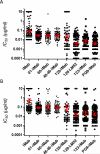
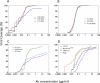
Results: Some of our biAbs neutralized 100% of HIV-1 strains tested in vitro at clinically achievable concentrations. Distinct neutralization patterns were observed in this panel of biAbs. Those biAbs with specificity for the CD4-binding site on gp120 demonstrated 100% breadth, as well as slightly improved potency compared with iMab. In contrast, biAbs with specificity for the V1-V2 apex epitope or the V3-glycan epitope on gp120 demonstrated dramatically improved potency; some showed limited gain in neutralization breadth, whereas others (eg, PGT128-LM52 and 123-iMab) improved to 100% breadth.
Conclusion: Our data suggest that this panel of iMab-based biAbs could be used to probe the parameters for potent HIV-1 neutralization. Moreover, a few of these biAbs warrant further studies and possibly clinical development.
Figures

Similar articles
-
Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13540-5. doi: 10.1073/pnas.1304985110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878231 Free PMC article.
-
A Bispecific Antibody That Simultaneously Recognizes the V2- and V3-Glycan Epitopes of the HIV-1 Envelope Glycoprotein Is Broader and More Potent than Its Parental Antibodies.mBio. 2020 Jan 14;11(1):e03080-19. doi: 10.1128/mBio.03080-19. mBio. 2020. PMID: 31937648 Free PMC article.
-
Engineering and characterising a novel, highly potent bispecific antibody iMab-CAP256 that targets HIV-1.Retrovirology. 2019 Nov 8;16(1):31. doi: 10.1186/s12977-019-0493-y. Retrovirology. 2019. PMID: 31703699 Free PMC article.
-
Engineering multi-specific antibodies against HIV-1.Retrovirology. 2018 Aug 29;15(1):60. doi: 10.1186/s12977-018-0439-9. Retrovirology. 2018. PMID: 30157871 Free PMC article. Review.
-
HIV Broadly Neutralizing Antibodies: VRC01 and Beyond.Adv Exp Med Biol. 2018;1075:53-72. doi: 10.1007/978-981-13-0484-2_3. Adv Exp Med Biol. 2018. PMID: 30030789 Review.
Cited by
-
An update on antiviral antibody-based biopharmaceuticals.Int Immunopharmacol. 2020 Sep;86:106760. doi: 10.1016/j.intimp.2020.106760. Epub 2020 Jul 6. Int Immunopharmacol. 2020. PMID: 32645633 Free PMC article. Review.
-
Conditionally Replicating Vectors Mobilize Chimeric Antigen Receptors against HIV.Mol Ther Methods Clin Dev. 2020 Sep 28;19:285-294. doi: 10.1016/j.omtm.2020.09.014. eCollection 2020 Dec 11. Mol Ther Methods Clin Dev. 2020. PMID: 33102620 Free PMC article.
-
Ibalizumab Targeting CD4 Receptors, An Emerging Molecule in HIV Therapy.Front Microbiol. 2017 Nov 27;8:2323. doi: 10.3389/fmicb.2017.02323. eCollection 2017. Front Microbiol. 2017. PMID: 29230203 Free PMC article. Review.
-
GSK3732394: a Multi-specific Inhibitor of HIV Entry.J Virol. 2019 Sep 30;93(20):e00907-19. doi: 10.1128/JVI.00907-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375580 Free PMC article.
-
Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection.Antimicrob Agents Chemother. 2019 May 24;63(6):e00110-19. doi: 10.1128/AAC.00110-19. Print 2019 Jun. Antimicrob Agents Chemother. 2019. PMID: 30885900 Free PMC article. Review.
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England Journal of Medicine. 2013 Aug 2;367(5):423–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

