Reward magnitude tracking by neural populations in ventral striatum
- PMID: 27789262
- PMCID: PMC5619692
- DOI: 10.1016/j.neuroimage.2016.10.036
Reward magnitude tracking by neural populations in ventral striatum
Abstract
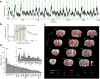
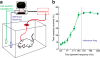
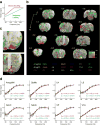
Evaluation of the magnitudes of intrinsically rewarding stimuli is essential for assigning value and guiding behavior. By combining parametric manipulation of a primary reward, medial forebrain bundle (MFB) microstimulation, with functional magnetic imaging (fMRI) in rodents, we delineated a broad network of structures activated by behaviorally characterized levels of rewarding stimulation. Correlation of psychometric behavioral measurements with fMRI response magnitudes revealed regions whose activity corresponded closely to the subjective magnitude of rewards. The largest and most reliable focus of reward magnitude tracking was observed in the shell region of the nucleus accumbens (NAc). Although the nonlinear nature of neurovascular coupling complicates interpretation of fMRI findings in precise neurophysiological terms, reward magnitude tracking was not observed in vascular compartments and could not be explained by saturation of region-specific hemodynamic responses. In addition, local pharmacological inactivation of NAc changed the profile of animals' responses to rewards of different magnitudes without altering mean reward response rates, further supporting a hypothesis that neural population activity in this region contributes to assessment of reward magnitudes.
Keywords: BOLD fMRI; Microstimulation; Nucleus accumbens; Reward.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

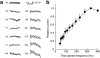
Similar articles
-
The neural substrates for the rewarding and dopamine-releasing effects of medial forebrain bundle stimulation have partially discrepant frequency responses.Behav Brain Res. 2016 Jan 15;297:345-58. doi: 10.1016/j.bbr.2015.10.029. Epub 2015 Oct 23. Behav Brain Res. 2016. PMID: 26477378
-
Synchronized electrical stimulation of the rat medial forebrain bundle and perforant pathway generates an additive BOLD response in the nucleus accumbens and prefrontal cortex.Neuroimage. 2013 Aug 15;77:14-25. doi: 10.1016/j.neuroimage.2013.03.046. Epub 2013 Apr 2. Neuroimage. 2013. PMID: 23558098
-
The effects of feeding and rewarding brain stimulation on lateral hypothalamic unit activity in freely moving rats.Brain Res. 1984 Nov 26;322(2):201-11. doi: 10.1016/0006-8993(84)90110-0. Brain Res. 1984. PMID: 6150748
-
Antidepressant-like Effects of Medial Forebrain Bundle Deep Brain Stimulation in Rats are not Associated With Accumbens Dopamine Release.Brain Stimul. 2015 Jul-Aug;8(4):708-13. doi: 10.1016/j.brs.2015.02.007. Epub 2015 Mar 10. Brain Stimul. 2015. PMID: 25835354
-
Anatomic Review of the Ventral Capsule/Ventral Striatum and the Nucleus Accumbens to Guide Target Selection for Deep Brain Stimulation for Obsessive-Compulsive Disorder.World Neurosurg. 2019 Jun;126:1-10. doi: 10.1016/j.wneu.2019.01.254. Epub 2019 Feb 18. World Neurosurg. 2019. PMID: 30790738 Review.
Cited by
-
Striatal responsiveness to reward under threat-of-shock and working memory load: A preliminary study.Brain Behav. 2019 Oct;9(10):e01397. doi: 10.1002/brb3.1397. Epub 2019 Sep 26. Brain Behav. 2019. PMID: 31557426 Free PMC article.
-
Calcium-dependent molecular fMRI using a magnetic nanosensor.Nat Nanotechnol. 2018 Jun;13(6):473-477. doi: 10.1038/s41565-018-0092-4. Epub 2018 Apr 30. Nat Nanotechnol. 2018. PMID: 29713073 Free PMC article.
-
Customizing MRI-Compatible Multifunctional Neural Interfaces through Fiber Drawing.Adv Funct Mater. 2021 Oct 20;31(43):2104857. doi: 10.1002/adfm.202104857. Epub 2021 Aug 6. Adv Funct Mater. 2021. PMID: 34924913 Free PMC article.
-
Local and global consequences of reward-evoked striatal dopamine release.Nature. 2020 Apr;580(7802):239-244. doi: 10.1038/s41586-020-2158-3. Epub 2020 Apr 1. Nature. 2020. PMID: 32269346 Free PMC article.
-
Imbalanced sensitivities to primary and secondary rewards in internet gaming disorder.J Behav Addict. 2021 Oct 29;10(4):990-1004. doi: 10.1556/2006.2021.00072. Online ahead of print. J Behav Addict. 2021. PMID: 34727087 Free PMC article.
References
-
- Albertin SV, Mulder AB, Tabuchi E, Zugaro MB, Wiener SI. Lesions of the medial shell of the nucleus accumbens impair rats in finding larger rewards, but spare reward-seeking behavior. Behav Brain Res. 2000;117:173–183. - PubMed
-
- Buonocore MH, Gao L. Ghost artifact reduction for echo planar imaging using image phase correction. Magn Reson Med. 1997;38:89–100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

