Means to the ends: The role of telomeres and telomere processing machinery in metastasis
- PMID: 27768860
- PMCID: PMC5138103
- DOI: 10.1016/j.bbcan.2016.10.005
Means to the ends: The role of telomeres and telomere processing machinery in metastasis
Abstract
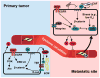
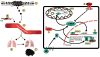
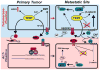
Despite significant clinical advancements, cancer remains a leading cause of mortality throughout the world due largely to the process of metastasis and the dissemination of cancer cells from their primary tumor of origin to distant secondary sites. The clinical burden imposed by metastasis is further compounded by a paucity of information regarding the factors that mediate metastatic progression. Linear chromosomes are capped by structures known as telomeres, which dictate cellular lifespan in humans by shortening progressively during successive cell divisions. Although telomere shortening occurs in nearly all somatic cells, telomeres may be elongated via two seemingly disjoint pathways: (i) telomerase-mediated extension, and (ii) homologous recombination-based alternative lengthening of telomeres (ALT). Both telomerase and ALT are activated in various human cancers, with more recent evidence implicating both pathways as potential mediators of metastasis. Here we review the known roles of telomere homeostasis in metastasis and posit a mechanism whereby metastatic activity is determined by a dynamic fluctuation between ALT and telomerase, as opposed to the mere activation of a generic telomere elongation program. Additionally, the pleiotropic nature of the telomere processing machinery makes it an attractive therapeutic target for metastasis, and as such, we also explore the therapeutic implications of our proposed mechanism.
Keywords: Alternative lengthening of telomeres; DNA damage; DNA repair; Epithelial-mesenchymal transition; Metastasis; Signal transduction; Telomere homeostasis; Telomeres.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
-
Beginning at the ends: telomere and telomere-based cancer therapeutics.Mol Genet Genomics. 2024 Dec 6;300(1):1. doi: 10.1007/s00438-024-02206-6. Mol Genet Genomics. 2024. PMID: 39638969 Review.
-
Multiomics analysis reveals the involvement of NET1 in tumour immune regulation and malignant progression.Sci Rep. 2025 Jan 2;15(1):56. doi: 10.1038/s41598-024-83714-8. Sci Rep. 2025. PMID: 39747410 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
Cited by
-
Telomere as a Therapeutic Target in Dedifferentiated Liposarcoma.Cancers (Basel). 2022 May 25;14(11):2624. doi: 10.3390/cancers14112624. Cancers (Basel). 2022. PMID: 35681604 Free PMC article.
-
Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism.Int J Mol Sci. 2021 Oct 14;22(20):11101. doi: 10.3390/ijms222011101. Int J Mol Sci. 2021. PMID: 34681758 Free PMC article.
-
Amplification and Quantitation of Telomeric Extrachromosomal Circles.Bio Protoc. 2023 Mar 5;13(5):e4627. doi: 10.21769/BioProtoc.4627. eCollection 2023 Mar 5. Bio Protoc. 2023. PMID: 36908640 Free PMC article.
-
Beginning at the ends: telomeres and human disease.F1000Res. 2018 May 1;7:F1000 Faculty Rev-524. doi: 10.12688/f1000research.14068.1. eCollection 2018. F1000Res. 2018. PMID: 29770205 Free PMC article. Review.
-
Targeting Telomere Dynamics as an Effective Approach for the Development of Cancer Therapeutics.Int J Nanomedicine. 2024 Apr 29;19:3805-3825. doi: 10.2147/IJN.S448556. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38708177 Free PMC article. Review.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. - PubMed
-
- Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

