Diverted recycling-Shigella subversion of Rabs
- PMID: 27763815
- PMCID: PMC5997151
- DOI: 10.1080/21541248.2016.1240494
Diverted recycling-Shigella subversion of Rabs
Abstract
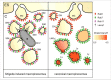
Small GTPases of the Rab protein family control intracellular vesicular trafficking to allow their communication and maintenance. It is a common strategy for intracellular bacteria to exploit these pathways to shape their respective niches for survival. The subversion of Rabs for the generation of an intracellular environment favoring the pathogen has been described almost exclusively for intracellular bacteria that reside within bacterial containing vacuoles (BCVs). However, less is known about Rab subversion for bacteria that rupture the BCV to reach the host cytoplasm. Here, we provide recent examples of Rab targeting by both groups of intracellular bacteria with a special focus on Shigella, the causative agent of bacillary dysentery. Shigella recruits Rab11, the hallmark of the perinuclear recycling compartment to in situ formed macropinosomes at the entry foci via the bacterial effector IpgD. This leads to efficient BCV rupture and cytosolic escape. We discuss the concept of diverted recycling through host Rab GTPases that emerges as a novel pathogen strategy.
Keywords: Rab subversion; Rab11; Shigella flexneri; bacterial containing vacuole; intracellular bacteria; macropinocytosis; vacuolar rupture; vesicular traffic.
Figures
Similar articles
-
Shigella hijacks the exocyst to cluster macropinosomes for efficient vacuolar escape.PLoS Pathog. 2020 Aug 31;16(8):e1008822. doi: 10.1371/journal.ppat.1008822. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866204 Free PMC article.
-
Shigella subverts the host recycling compartment to rupture its vacuole.Cell Host Microbe. 2014 Oct 8;16(4):517-30. doi: 10.1016/j.chom.2014.09.005. Cell Host Microbe. 2014. PMID: 25299335
-
Cytosolic Access of Intracellular Bacterial Pathogens: The Shigella Paradigm.Front Cell Infect Microbiol. 2016 Apr 5;6:35. doi: 10.3389/fcimb.2016.00035. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27092296 Free PMC article. Review.
-
Macropinosomes are Key Players in Early Shigella Invasion and Vacuolar Escape in Epithelial Cells.PLoS Pathog. 2016 May 16;12(5):e1005602. doi: 10.1371/journal.ppat.1005602. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27182929 Free PMC article.
-
Taking control: Hijacking of Rab GTPases by intracellular bacterial pathogens.Small GTPases. 2018 Mar 4;9(1-2):182-191. doi: 10.1080/21541248.2017.1336192. Epub 2017 Jul 5. Small GTPases. 2018. PMID: 28632996 Free PMC article. Review.
Cited by
-
The recycling endosome and bacterial pathogens.Cell Microbiol. 2018 Jul;20(7):e12857. doi: 10.1111/cmi.12857. Epub 2018 May 30. Cell Microbiol. 2018. PMID: 29748997 Free PMC article. Review.
-
The Endosomal Recycling Pathway-At the Crossroads of the Cell.Int J Mol Sci. 2020 Aug 23;21(17):6074. doi: 10.3390/ijms21176074. Int J Mol Sci. 2020. PMID: 32842549 Free PMC article. Review.
-
Modification of phosphoinositides by the Shigella effector IpgD during host cell infection.Front Cell Infect Microbiol. 2022 Oct 27;12:1012533. doi: 10.3389/fcimb.2022.1012533. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389142 Free PMC article. Review.
-
Selective autophagy of RIPosomes maintains innate immune homeostasis during bacterial infection.EMBO J. 2022 Dec 1;41(23):e111289. doi: 10.15252/embj.2022111289. Epub 2022 Oct 11. EMBO J. 2022. PMID: 36221902 Free PMC article.
References
-
- Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature 2007; 449:827-34; PMID:17943119; https://doi.org/10.1038/nature06247 - DOI - PubMed
-
- Cossart P, Craig RR. Manipulation of host membrane machinery by bacterial pathogens. Curr Opin Cell Biol 2010; 22:547-54; PMID:20542678; https://doi.org/10.1016/j.ceb.2010.05.006 - DOI - PMC - PubMed
-
- Sherwood RK, Roy CR. A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host Microbe 2013; 14:256-68; https://doi.org/10.1016/j.chom.2013.08.010 - DOI - PMC - PubMed
-
- Stein MP, Müller MP, Wandinger-Ness A. Bacterial pathogens commandeer Rab GTPases to establish intracellular niches. Traffic 2012; 13:1565-88; PMID:22901006; https://doi.org/10.1111/tra.12000 - DOI - PMC - PubMed
-
- Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015; 128:3171-6; PMID:26272922; https://doi.org/10.1242/jcs.166074 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
