Diadenosine tetraphosphate (Ap4A) inhibits ATP-induced excitotoxicity: a neuroprotective strategy for traumatic spinal cord injury treatment
- PMID: 27761681
- PMCID: PMC5334201
- DOI: 10.1007/s11302-016-9541-4
Diadenosine tetraphosphate (Ap4A) inhibits ATP-induced excitotoxicity: a neuroprotective strategy for traumatic spinal cord injury treatment
Abstract
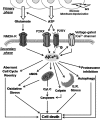
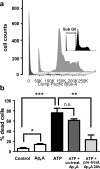
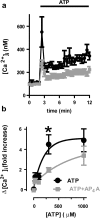
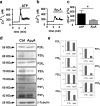
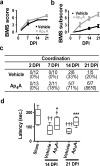
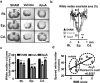
Reducing cell death during the secondary injury is a major priority in the development of a cure for traumatic spinal cord injury (SCI). One of the earliest processes that follow SCI is the excitotoxicity resulting from the massive release of excitotoxicity mediators, including ATP, which induce an excessive and/or prolonged activation of their receptors and a deregulation of the calcium homeostasis. Diadenosine tetraphosphate (Ap4A) is an endogenous purinergic agonist, present in both extracellular and intracellular fluids, with promising cytoprotective effects in different diseases including neurodegenerative processes. In a search for efficient neuroprotective strategies for SCI, we have tested the capability of Ap4A to reduce the excitotoxic death mediated by the ATP-induced deregulation of calcium homeostasis and its consequences on tissue preservation and functional recovery in a mouse model of moderate contusive SCI. Our analyses with the murine neural cell line Neuro2a demonstrate that treatment with Ap4A reduces ATP-dependent excitotoxic death by both lowering the intracellular calcium response and decreasing the expression of specific purinergic receptors. Follow-up analyses in a mouse model of contusive SCI showed that acute administration of Ap4A following SCI reduces tissue damage and improves motor function recovery. These results suggest that Ap4A cytoprotection results from a decrease of the purinergic tone preventing the effects of a massive release of ATP after SCI, probably together with a direct induction of anti-apoptotic and pro-survival pathways via activation of P2Y2 proposed in previous studies. In conclusion, Ap4A may be a good candidate for an SCI therapy, particularly to reduce excitotoxicity in combination with other modulators and/or inhibitors of the excitotoxic process that are being tested.
Keywords: Apoptosis; Diadenosine; Excitotoxicity; Intracellular calcium; Mouse model; Neuro-2a; Neuroprotection; Secondary injury; Spinal cord injury; Tissue damage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
MicroRNA-135a-5p reduces P2X7 -dependent rise in intracellular calcium and protects against excitotoxicity.J Neurochem. 2019 Oct;151(1):116-130. doi: 10.1111/jnc.14700. Epub 2019 May 9. J Neurochem. 2019. PMID: 30924927
-
Acute administration of ucf-101 ameliorates the locomotor impairments induced by a traumatic spinal cord injury.Neuroscience. 2015 Aug 6;300:404-17. doi: 10.1016/j.neuroscience.2015.05.036. Epub 2015 May 22. Neuroscience. 2015. PMID: 26004679
-
Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain.J Neurosci. 2003 Aug 27;23(21):7958-65. doi: 10.1523/JNEUROSCI.23-21-07958.2003. J Neurosci. 2003. PMID: 12944527 Free PMC article.
-
The therapeutic role of interleukin-10 after spinal cord injury.J Neurotrauma. 2013 Aug 1;30(15):1311-24. doi: 10.1089/neu.2012.2651. Epub 2013 Jul 18. J Neurotrauma. 2013. PMID: 23731227 Review.
-
Riluzole for acute traumatic spinal cord injury: a promising neuroprotective treatment strategy.World Neurosurg. 2014 May-Jun;81(5-6):825-9. doi: 10.1016/j.wneu.2013.01.001. Epub 2013 Jan 4. World Neurosurg. 2014. PMID: 23295632 Review.
Cited by
-
Intracerebroventricular Delivery of Human Umbilical Cord Mesenchymal Stem Cells as a Promising Therapy for Repairing the Spinal Cord Injury Induced by Kainic Acid.Stem Cell Rev Rep. 2020 Feb;16(1):167-180. doi: 10.1007/s12015-019-09934-y. Stem Cell Rev Rep. 2020. PMID: 31760626
-
Purinergic Signalling: Therapeutic Developments.Front Pharmacol. 2017 Sep 25;8:661. doi: 10.3389/fphar.2017.00661. eCollection 2017. Front Pharmacol. 2017. PMID: 28993732 Free PMC article. Review.
-
Corrigendum: Purinergic signaling systems across comparative models of spinal cord injury.Neural Regen Res. 2023 Mar;18(3):689-696. doi: 10.4103/1673-5374.350234. Neural Regen Res. 2023. PMID: 36018196 Free PMC article.
-
The Alarmone Diadenosine Tetraphosphate as a Cosubstrate for Protein AMPylation.Angew Chem Int Ed Engl. 2023 Feb 13;62(8):e202213279. doi: 10.1002/anie.202213279. Epub 2023 Jan 16. Angew Chem Int Ed Engl. 2023. PMID: 36524454 Free PMC article.
-
Bayesian functional analysis for untargeted metabolomics data with matching uncertainty and small sample sizes.Brief Bioinform. 2024 Mar 27;25(3):bbae141. doi: 10.1093/bib/bbae141. Brief Bioinform. 2024. PMID: 38581417 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

