CREB decreases astrocytic excitability by modifying subcellular calcium fluxes via the sigma-1 receptor
- PMID: 27761593
- PMCID: PMC11107612
- DOI: 10.1007/s00018-016-2397-5
CREB decreases astrocytic excitability by modifying subcellular calcium fluxes via the sigma-1 receptor
Abstract
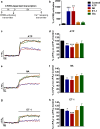
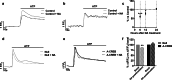
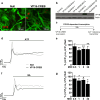
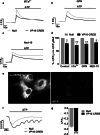
Astrocytic excitability relies on cytosolic calcium increases as a key mechanism, whereby astrocytes contribute to synaptic transmission and hence learning and memory. While it is a cornerstone of neurosciences that experiences are remembered, because transmitters activate gene expression in neurons, long-term adaptive astrocyte plasticity has not been described. Here, we investigated whether the transcription factor CREB mediates adaptive plasticity-like phenomena in astrocytes. We found that activation of CREB-dependent transcription reduced the calcium responses induced by ATP, noradrenaline, or endothelin-1. As to the mechanism, expression of VP16-CREB, a constitutively active CREB mutant, had no effect on basal cytosolic calcium levels, extracellular calcium entry, or calcium mobilization from lysosomal-related acidic stores. Rather, VP16-CREB upregulated sigma-1 receptor expression thereby increasing the release of calcium from the endoplasmic reticulum and its uptake by mitochondria. Sigma-1 receptor was also upregulated in vivo upon VP16-CREB expression in astrocytes. We conclude that CREB decreases astrocyte responsiveness by increasing calcium signalling at the endoplasmic reticulum-mitochondria interface, which might be an astrocyte-based form of long-term depression.
Keywords: CEPIA indicators; Calcium signalling; Endoplasmic reticulum; MCU; Mitochondria; Mitochondria-associated membranes; VP16-CREB.
Figures


Similar articles
-
Cyclic AMP response element-binding protein (CREB) transcription factor in astrocytic synaptic communication.Front Synaptic Neurosci. 2023 Jan 4;14:1059918. doi: 10.3389/fnsyn.2022.1059918. eCollection 2022. Front Synaptic Neurosci. 2023. PMID: 36685081 Free PMC article. Review.
-
ATP and noradrenaline activate CREB in astrocytes via noncanonical Ca(2+) and cyclic AMP independent pathways.Glia. 2012 Sep;60(9):1330-44. doi: 10.1002/glia.22352. Epub 2012 May 16. Glia. 2012. PMID: 22593004
-
CREB Regulates Distinct Adaptive Transcriptional Programs in Astrocytes and Neurons.Sci Rep. 2017 Jul 25;7(1):6390. doi: 10.1038/s41598-017-06231-x. Sci Rep. 2017. PMID: 28743894 Free PMC article.
-
Calcium signalling in astroglia.Mol Cell Endocrinol. 2012 Apr 28;353(1-2):45-56. doi: 10.1016/j.mce.2011.08.039. Epub 2011 Sep 10. Mol Cell Endocrinol. 2012. PMID: 21945602 Review.
-
Involvement of sigma-1 receptor in astrocyte activation induced by methamphetamine via up-regulation of its own expression.J Neuroinflammation. 2015 Feb 17;12:29. doi: 10.1186/s12974-015-0250-7. J Neuroinflammation. 2015. PMID: 25889537 Free PMC article.
Cited by
-
Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function.Commun Biol. 2020 Nov 17;3(1):682. doi: 10.1038/s42003-020-01408-z. Commun Biol. 2020. PMID: 33203971 Free PMC article.
-
Glutamate Carrier Involvement in Mitochondrial Dysfunctioning in the Brain White Matter.Front Mol Biosci. 2020 Jul 21;7:151. doi: 10.3389/fmolb.2020.00151. eCollection 2020. Front Mol Biosci. 2020. PMID: 32793632 Free PMC article.
-
Sex-dependent calcium hyperactivity due to lysosomal-related dysfunction in astrocytes from APOE4 versus APOE3 gene targeted replacement mice.Mol Neurodegener. 2020 Jun 9;15(1):35. doi: 10.1186/s13024-020-00382-8. Mol Neurodegener. 2020. PMID: 32517777 Free PMC article.
-
Cyclic AMP response element-binding protein (CREB) transcription factor in astrocytic synaptic communication.Front Synaptic Neurosci. 2023 Jan 4;14:1059918. doi: 10.3389/fnsyn.2022.1059918. eCollection 2022. Front Synaptic Neurosci. 2023. PMID: 36685081 Free PMC article. Review.
-
The effectiveness of Bacopa monnieri (Linn.) Wettst. as a nootropic, neuroprotective, or antidepressant supplement: analysis of the available clinical data.Sci Rep. 2021 Jan 12;11(1):596. doi: 10.1038/s41598-020-80045-2. Sci Rep. 2021. PMID: 33436817 Free PMC article.
References
-
- Li H, Wang X, Zhang N, Gottipati MK, Parpura V, Ding S. Imaging of mitochondrial Ca2+ dynamics in astrocytes using cell-specific mitochondria-targeted GCaMP5G/6s: mitochondrial Ca2+ uptake and cytosolic Ca2+ availability via the endoplasmic reticulum store. Cell Calcium. 2014;56:457–466. doi: 10.1016/j.ceca.2014.09.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

