MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults
- PMID: 27760761
- PMCID: PMC5216266
- DOI: 10.1182/blood-2016-07-729756
MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults
Abstract
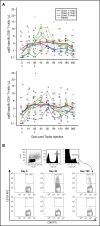
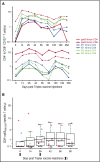
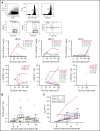
Attenuated poxvirus modified vaccinia Ankara (MVA) is a useful viral-based vaccine for clinical investigation, because of its excellent safety profile and property of inducing potent immune responses against recombinant (r) antigens. We developed Triplex by constructing an rMVA encoding 3 immunodominant cytomegalovirus (CMV) antigens, which stimulates a host antiviral response: UL83 (pp65), UL123 (IE1-exon4), and UL122 (IE2-exon5). We completed the first clinical evaluation of the Triplex vaccine in 24 healthy adults, with or without immunity to CMV and vaccinia virus (previous DryVax smallpox vaccination). Three escalating dose levels (DL) were administered IM in 8 subjects/DL, with an identical booster injection 28 days later and 1-year follow-up. Vaccinations at all DL were safe with no dose-limiting toxicities. No vaccine-related serious adverse events were documented. Local and systemic reactogenicity was transient and self-limiting. Robust, functional, and durable Triplex-driven expansions of CMV-specific T cells were detected by measuring T-cell surface levels of 4-1BB (CD137), binding to CMV-specific HLA multimers, and interferon-γ production. Marked and durable CMV-specific T-cell responses were also detected in Triplex-vaccinated CMV-seronegatives, and in DryVax-vaccinated subjects. Long-lived memory effector phenotype, associated with viral control during CMV primary infection, was predominantly found on the membrane of CMV-specific and functional T cells, whereas off-target vaccine responses activating memory T cells from the related herpesvirus Epstein-Barr virus remained undetectable. Combined safety and immunogenicity results of MVA in allogeneic hematopoietic stem cell transplant (HCT) recipients and Triplex in healthy adults motivated the initiation of a placebo-controlled multicenter trial of Triplex in HCT patients. This trial was registered at www.clinicaltrials.gov as #NCT02506933.
© 2017 by The American Society of Hematology.
Figures
Similar articles
-
Attenuated poxvirus expressing three immunodominant CMV antigens as a vaccine strategy for CMV infection.J Clin Virol. 2006 Mar;35(3):324-31. doi: 10.1016/j.jcv.2005.09.018. Epub 2006 Jan 4. J Clin Virol. 2006. PMID: 16388983
-
Hematopoietic stem cell donor vaccination with cytomegalovirus triplex augments frequencies of functional and durable cytomegalovirus-specific T cells in the recipient: A novel strategy to limit antiviral prophylaxis.Am J Hematol. 2023 Apr;98(4):588-597. doi: 10.1002/ajh.26824. Epub 2023 Jan 11. Am J Hematol. 2023. PMID: 36594185 Free PMC article.
-
Poxvirus Vectored Cytomegalovirus Vaccine to Prevent Cytomegalovirus Viremia in Transplant Recipients: A Phase 2, Randomized Clinical Trial.Ann Intern Med. 2020 Mar 3;172(5):306-316. doi: 10.7326/M19-2511. Epub 2020 Feb 11. Ann Intern Med. 2020. PMID: 32040960 Free PMC article. Clinical Trial.
-
Vaccine properties of a novel marker gene-free recombinant modified vaccinia Ankara expressing immunodominant CMV antigens pp65 and IE1.Vaccine. 2007 Jan 22;25(6):1132-41. doi: 10.1016/j.vaccine.2006.09.067. Epub 2006 Oct 6. Vaccine. 2007. PMID: 17049414 Free PMC article.
-
The next generation recombinant human cytomegalovirus vaccine candidates-beyond gB.Vaccine. 2012 Nov 19;30(49):6980-90. doi: 10.1016/j.vaccine.2012.09.056. Epub 2012 Oct 3. Vaccine. 2012. PMID: 23041121 Review.
Cited by
-
Seropositivity for pathogens associated with chronic infections is a risk factor for all-cause mortality in the elderly: findings from the Memory and Morbidity in Augsburg Elderly (MEMO) Study.Geroscience. 2020 Oct;42(5):1365-1376. doi: 10.1007/s11357-020-00216-x. Epub 2020 Jul 9. Geroscience. 2020. PMID: 32648237 Free PMC article.
-
Pre-clinical data supporting immunotherapy for HIV using CMV-HIV-specific CAR T cells with CMV vaccine.Mol Ther Methods Clin Dev. 2022 Apr 13;25:344-359. doi: 10.1016/j.omtm.2022.04.007. eCollection 2022 Jun 9. Mol Ther Methods Clin Dev. 2022. PMID: 35573050 Free PMC article.
-
Neutralization of Human Cytomegalovirus Entry into Fibroblasts and Epithelial Cells.Vaccines (Basel). 2017 Oct 31;5(4):39. doi: 10.3390/vaccines5040039. Vaccines (Basel). 2017. PMID: 29088098 Free PMC article. Review.
-
A Novel Monoclonal Antibody Against a Modified Vaccinia Ankara (MVA) Envelope Protein as a Tool for MVA Virus Titration by Flow Cytometry.Viruses. 2024 Oct 17;16(10):1628. doi: 10.3390/v16101628. Viruses. 2024. PMID: 39459960 Free PMC article.
-
Development of a Synthetic Poxvirus-Based SARS-CoV-2 Vaccine.bioRxiv [Preprint]. 2020 Jul 2:2020.07.01.183236. doi: 10.1101/2020.07.01.183236. bioRxiv. 2020. Update in: Nat Commun. 2020 Nov 30;11(1):6121. doi: 10.1038/s41467-020-19819-1 PMID: 32637957 Free PMC article. Updated. Preprint.
References
-
- Schmidt-Hieber M, Labopin M, Beelen D, et al. . CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359-3364. - PubMed
-
- Lacey SF, La Rosa C, Zhou W, et al. . Functional comparison of T cells recognizing cytomegalovirus pp65 and intermediate-early antigen polypeptides in hematopoietic stem-cell transplant and solid organ transplant recipients. J Infect Dis. 2006;194(10):1410-1421. - PubMed
-
- Gabanti E, Lilleri D, Ripamonti F, et al. . Reconstitution of human cytomegalovirus-specific CD4+ T cells is critical for control of virus reactivation in hematopoietic stem cell transplant recipients but does not prevent organ infection. Biol Blood Marrow Transplant. 2015;21(12):2192-2202. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

