Adaptation in Bacillus cereus: From Stress to Disease
- PMID: 27757102
- PMCID: PMC5047918
- DOI: 10.3389/fmicb.2016.01550
Adaptation in Bacillus cereus: From Stress to Disease
Abstract
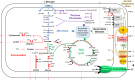
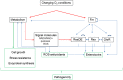
Bacillus cereus is a food-borne pathogen that causes diarrheal disease in humans. After ingestion, B. cereus experiences in the human gastro-intestinal tract abiotic physical variables encountered in food, such as acidic pH in the stomach and changing oxygen conditions in the human intestine. B. cereus responds to environmental changing conditions (stress) by reversibly adjusting its physiology to maximize resource utilization while maintaining structural and genetic integrity by repairing and minimizing damage to cellular infrastructure. As reviewed in this article, B. cereus adapts to acidic pH and changing oxygen conditions through diverse regulatory mechanisms and then exploits its metabolic flexibility to grow and produce enterotoxins. We then focus on the intricate link between metabolism, redox homeostasis, and enterotoxins, which are recognized as important contributors of food-borne disease.
Keywords: Bacillus cereus; acidic pH; metabolism; oxygen sensing; redox homeostasis.
Figures

Similar articles
-
The effect of selected factors on the survival of Bacillus cereus in the human gastrointestinal tract.Microb Pathog. 2015 May;82:7-14. doi: 10.1016/j.micpath.2015.03.015. Epub 2015 Mar 17. Microb Pathog. 2015. PMID: 25794697 Review.
-
Enterotoxin production by Bacillus cereus under gastrointestinal conditions and their immunological detection by commercially available kits.Foodborne Pathog Dis. 2012 Dec;9(12):1130-6. doi: 10.1089/fpd.2012.1230. Foodborne Pathog Dis. 2012. PMID: 23237409
-
Reducing activity, glucose metabolism and acid tolerance response of Bacillus cereus grown at various pH and oxydo-reduction potential levels.Food Microbiol. 2015 Apr;46:314-321. doi: 10.1016/j.fm.2014.07.007. Epub 2014 Aug 21. Food Microbiol. 2015. PMID: 25475301
-
Deciphering the interactions between the Bacillus cereus linear plasmid, pBClin15, and its host by high-throughput comparative proteomics.J Proteomics. 2016 Sep 2;146:25-33. doi: 10.1016/j.jprot.2016.06.022. Epub 2016 Jun 16. J Proteomics. 2016. PMID: 27321915
-
Prevalence and expression of enterotoxins in Bacillus cereus and other Bacillus spp., a literature review.Antonie Van Leeuwenhoek. 2000 May;77(4):393-9. doi: 10.1023/a:1002706906154. Antonie Van Leeuwenhoek. 2000. PMID: 10959569 Review.
Cited by
-
Sporulation is dispensable for the vegetable-associated life cycle of the human pathogen Bacillus cereus.Microb Biotechnol. 2021 Jul;14(4):1550-1565. doi: 10.1111/1751-7915.13816. Epub 2021 May 6. Microb Biotechnol. 2021. PMID: 33955675 Free PMC article.
-
Genome Sequence of a Moderately Halophilic Bacillus cereus Strain, TS2, Isolated from Saltern Sediments.Microbiol Resour Announc. 2018 Aug 16;7(6):e00873-18. doi: 10.1128/MRA.00873-18. eCollection 2018 Aug. Microbiol Resour Announc. 2018. PMID: 30533897 Free PMC article.
-
Listeria monocytogenes Requires the RsbX Protein To Prevent SigB Activation under Nonstressed Conditions.J Bacteriol. 2022 Jan 18;204(1):e0048621. doi: 10.1128/JB.00486-21. Epub 2021 Oct 25. J Bacteriol. 2022. PMID: 34694900 Free PMC article.
-
Characterization and Genetic Diversity of Bacillus cereus Strains Isolated from Baby Wipes.Microorganisms. 2022 Sep 3;10(9):1779. doi: 10.3390/microorganisms10091779. Microorganisms. 2022. PMID: 36144383 Free PMC article.
-
In vitro and in silico assessment of probiotic and functional properties of Bacillus subtilis DE111®.Front Microbiol. 2023 Jan 13;13:1101144. doi: 10.3389/fmicb.2022.1101144. eCollection 2022. Front Microbiol. 2023. PMID: 36713219 Free PMC article.
References
-
- Alvarez-Ordonez A., Fernandez A., Bernardo A., Lopez M. (2010a). Acid adaptation sensitizes Salmonella enterica serovar Typhimurium to osmotic and oxidative stresses. Arch. Lebensmittelhyg. 61 148–152. 10.2376/0003-925X-61-148 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

