Is it appropriate regarding patient preference to take Myrtol standardized enteric-coated soft capsules after a meal rather than at fasted state? A food-drug pharmacokinetic interaction study in healthy Chinese volunteers
- PMID: 27757023
- PMCID: PMC5055047
- DOI: 10.2147/PPA.S116823
Is it appropriate regarding patient preference to take Myrtol standardized enteric-coated soft capsules after a meal rather than at fasted state? A food-drug pharmacokinetic interaction study in healthy Chinese volunteers
Abstract
Background: According to prescribing information for Myrtol standardized enteric-coated soft capsules, the medicine should be taken on an empty stomach. Some patients may experience stomach discomfort after oral administration in fasted state and would prefer to take the medicine after a meal. However, there is no literature addressing the effect of meal on absorption of this drug; therefore, it is desirable to explore the feasibility of taking the capsule after a meal from pharmacokinetic perspective.
Methods: A gas chromatography coupled with triple quadruples mass spectrometry assay was established and validated for determining plasma concentrations of eucalyptol, a target component of Myrtol standardized capsules. A self-control clinical study was carried out in healthy male volunteers in fasted and fed states after a single oral dose of 300 mg capsules. Comparison of pharmacokinetic parameters in the two phases and bioequivalence evaluation were performed.
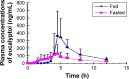
Results: The specificity, sensitivity, accuracy, and precision of the assay satisfied the requirements for biopharmaceutical analysis. Pharmacokinetic parameters of eucalyptol (fasted vs fed) were as follows: maximal plasma concentrations (Cmax) (167.60±114.69 vs 518.89±314.47 ng·mL-1), time of maximum concentration (Tmax) (3.7±1.1 vs 4.8±0.7 h), elimination half-life (T1/2) (3.2±1.4 vs 2.6±0.7 h), area under the plasma concentration-time curve (AUC0-t) (584.91±369.90 vs 1,271.61±605.82 ng·h·mL-1), and AUC0-∞ (690.36±467.26 vs 1,458.02±720.21 ng·h·mL-1). There was statistically significant difference in Cmax, AUC0-t, and AUC0-∞ between the two dosing methods (P<0.05). Pharmacokinetic parameters of eucalyptol given in fasted state in Chinese were comparable to those in Germany population. The 90% confidence intervals for the ratio of Cmax (18.4%~64.7%), AUC0-t (28.9%~68.5%), and AUC0-∞ (31.1%~68.4%) values for the test (fasted) and reference (fed) were beyond the Food and Drug Administration's acceptable range of 80%~125%. In addition, significant difference was obtained in Tmax (P<0.05).
Conclusion: Compared with dosing at fasted state, taking Myrtol standardized capsules after a meal achieves a delayed absorption rate and an increased absorption extent. The two dosing methods were not bioequivalent in this small study and, thus, not interchangeable. Patient preference and pharmacokinetic food-drug interaction issue should be balanced. Further clinical study is necessary to explore the clinical outcome of oral administration of Myrtol standardized capsules after or with meal.
Keywords: GC-MS; bioequivalence; dosing method; eucalyptol; food-drug interaction; gas chromatography mass spectrometry; herb; patient preference.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Pharmacokinetics and bioequivalence of Ezetimibe tablet versus Ezetrol®:an open-label, randomized, two-sequence crossover study in healthy Chinese subjects.BMC Pharmacol Toxicol. 2023 Feb 3;24(1):7. doi: 10.1186/s40360-023-00649-y. BMC Pharmacol Toxicol. 2023. PMID: 36737825 Free PMC article. Clinical Trial.
-
Bioequivalence of two esomeprazole magnesium enteric-coated formulations in healthy Chinese subjects.World J Clin Cases. 2020 Nov 26;8(22):5518-5528. doi: 10.12998/wjcc.v8.i22.5518. World J Clin Cases. 2020. PMID: 33344542 Free PMC article.
-
Single-dose bioequivalence assessment of two formulations of polysaccharide iron complex capsules in healthy adult male Chinese volunteers: A sequence-randomized, double-blind, two-way crossover study.Curr Ther Res Clin Exp. 2009 Apr;70(2):104-15. doi: 10.1016/j.curtheres.2009.04.006. Curr Ther Res Clin Exp. 2009. PMID: 24683222 Free PMC article.
-
Effects of food intake on the pharmacokinetic properties of mirabegron oral controlled-absorption system: a single-dose, randomized, crossover study in healthy adults.Clin Ther. 2013 Mar;35(3):333-41. doi: 10.1016/j.clinthera.2013.02.014. Clin Ther. 2013. PMID: 23497763 Clinical Trial.
-
Bioequivalence study of two formulations of flupirtine maleate capsules in healthy male Chinese volunteers under fasting and fed conditions.Drug Des Devel Ther. 2017 Dec 1;11:3441-3448. doi: 10.2147/DDDT.S149913. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29238169 Free PMC article. Clinical Trial.
Cited by
-
Volatile components in Yinchenzhufu decoction and their pharmacokinetics after oral administration in rats.RSC Adv. 2022 Jan 24;12(6):3287-3299. doi: 10.1039/d1ra08584k. eCollection 2022 Jan 24. RSC Adv. 2022. PMID: 35425370 Free PMC article.
References
-
- Ötles S, Senturk A. Food and drug interactions: a general review. Acta Sci Pol Technol Aliment. 2014;13:89–102. - PubMed
-
- Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet. 1999;36:233–254. - PubMed
-
- Joint Commission International Accreditation Standards for Hospitals. 5th edition. Oak Brook, IL: Joint Commission Resources; 2013.
-
- Matthys H, de Mey C, Carls C, Ryś A, Geib A, Wittig T. Efficacy and tolerability of myrtol standardized in acute bronchitis. A multi-centre, randomised, double-blind, placebo-controlled parallel group clinical trial vs. cefuroxime and ambroxol. Arzneimittelforschung. 2000;50:700–711. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

