Synaptic Vesicle Glycoprotein 2A Ligands in the Treatment of Epilepsy and Beyond
- PMID: 27752944
- PMCID: PMC5078162
- DOI: 10.1007/s40263-016-0384-x
Synaptic Vesicle Glycoprotein 2A Ligands in the Treatment of Epilepsy and Beyond
Abstract
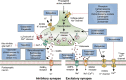
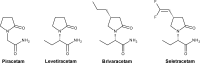
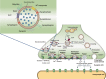
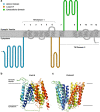
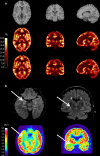
The synaptic vesicle glycoprotein SV2A belongs to the major facilitator superfamily (MFS) of transporters and is an integral constituent of synaptic vesicle membranes. SV2A has been demonstrated to be involved in vesicle trafficking and exocytosis, processes crucial for neurotransmission. The anti-seizure drug levetiracetam was the first ligand to target SV2A and displays a broad spectrum of anti-seizure activity in various preclinical models. Several lines of preclinical and clinical evidence, including genetics and protein expression changes, support an important role of SV2A in epilepsy pathophysiology. While the functional consequences of SV2A ligand binding are not fully elucidated, studies suggest that subsequent SV2A conformational changes may contribute to seizure protection. Conversely, the recently discovered negative SV2A modulators, such as UCB0255, counteract the anti-seizure effect of levetiracetam and display procognitive properties in preclinical models. More broadly, dysfunction of SV2A may also be involved in Alzheimer's disease and other types of cognitive impairment, suggesting potential novel therapies for levetiracetam and its congeners. Furthermore, emerging data indicate that there may be important roles for two other SV2 isoforms (SV2B and SV2C) in the pathogenesis of epilepsy, as well as other neurodegenerative diseases. Utilization of recently developed SV2A positron emission tomography ligands will strengthen and reinforce the pharmacological evidence that SV2A is a druggable target, and will provide a better understanding of its role in epilepsy and other neurological diseases, aiding in further defining the full therapeutic potential of SV2A modulation.
Conflict of interest statement
Compliance with Ethical Standards Funding Wolfgang Löscher’s initial animal studies on levetiracetam were supported by grants from UCB Pharma. No funding was obtained for preparing this current review. The open access payment for this article was funded by UCB Pharma. Conflict of interest Michel Gillard, Zara Sands, Rafal M. Kaminski, and Henrik Klitgaard are employees of UCB Pharma (Braine-l’Alleud, Belgium). Wolfgang Löscher has no conflicts of interest to declare.
Figures
Similar articles
-
The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam.Proc Natl Acad Sci U S A. 2004 Jun 29;101(26):9861-6. doi: 10.1073/pnas.0308208101. Epub 2004 Jun 21. Proc Natl Acad Sci U S A. 2004. PMID: 15210974 Free PMC article.
-
Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment.Epilepsia. 2016 Apr;57(4):538-48. doi: 10.1111/epi.13340. Epub 2016 Feb 26. Epilepsia. 2016. PMID: 26920914 Review.
-
No major role of common SV2A variation for predisposition or levetiracetam response in epilepsy.Epilepsy Res. 2009 Jan;83(1):44-51. doi: 10.1016/j.eplepsyres.2008.09.003. Epub 2008 Oct 31. Epilepsy Res. 2009. PMID: 18977120
-
Expression profile of synaptic vesicle glycoprotein 2A, B, and C paralogues in temporal neocortex tissue from patients with temporal lobe epilepsy (TLE).Mol Brain. 2022 May 16;15(1):45. doi: 10.1186/s13041-022-00931-w. Mol Brain. 2022. PMID: 35578248 Free PMC article.
-
Therapeutic Role of Synaptic Vesicle Glycoprotein 2A (SV2A) in Modulating Epileptogenesis.CNS Neurol Disord Drug Targets. 2017;16(4):463-471. doi: 10.2174/1871527316666170404115027. CNS Neurol Disord Drug Targets. 2017. PMID: 28393712 Review.
Cited by
-
SDI-118, a novel procognitive SV2A modulator: First-in-human randomized controlled trial including PET/fMRI assessment of target engagement.Front Pharmacol. 2023 Jan 17;13:1066447. doi: 10.3389/fphar.2022.1066447. eCollection 2022. Front Pharmacol. 2023. PMID: 36733374 Free PMC article.
-
Levetiracetam Attenuates Adolescent Stress-induced Behavioral and Electrophysiological Changes Associated With Schizophrenia in Adult Rats.Schizophr Bull. 2023 Jan 3;49(1):68-77. doi: 10.1093/schbul/sbac106. Schizophr Bull. 2023. PMID: 35988039 Free PMC article.
-
Levetiracetam Affects Differentially Presynaptic Proteins in Rat Cerebral Cortex.Front Cell Neurosci. 2017 Dec 11;11:389. doi: 10.3389/fncel.2017.00389. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29311825 Free PMC article.
-
Exploring with [18F]UCB-H the in vivo Variations in SV2A Expression through the Kainic Acid Rat Model of Temporal Lobe Epilepsy.Mol Imaging Biol. 2020 Oct;22(5):1197-1207. doi: 10.1007/s11307-020-01488-7. Mol Imaging Biol. 2020. PMID: 32206990 Free PMC article.
-
Lycium Barbarum Polysaccharides Improves Cognitive Functions in ICV-STZ-Induced Alzheimer's Disease Mice Model by Improving the Synaptic Structural Plasticity and Regulating IRS1/PI3K/AKT Signaling Pathway.Neuromolecular Med. 2024 Apr 23;26(1):15. doi: 10.1007/s12017-024-08784-3. Neuromolecular Med. 2024. PMID: 38653878
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

