Norovirus-Specific Memory T Cell Responses in Adult Human Donors
- PMID: 27752254
- PMCID: PMC5045929
- DOI: 10.3389/fmicb.2016.01570
Norovirus-Specific Memory T Cell Responses in Adult Human Donors
Abstract
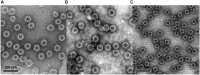
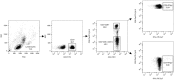
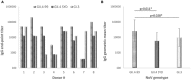
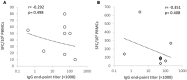
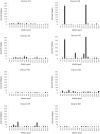
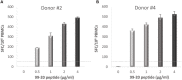
Norovirus (NoV) is a leading cause of acute gastroenteritis in people of all ages worldwide. NoV-specific serum antibodies which block the binding of NoV virus-like particles (VLPs) to the cell receptors have been thoroughly investigated. In contrast, only a few publications are available on the NoV capsid VP1 protein-specific T cell responses in humans naturally infected with the virus. Freshly isolated peripheral blood mononuclear cells of eight healthy adult human donors previously exposed to NoV were stimulated with purified VLPs derived from NoV GII.4-1999, GII.4-2012 (Sydney), and GI.3, and IFN-γ production was measured by an ELISPOT assay. In addition, 76 overlapping synthetic peptides spanning the entire 539-amino acid sequence of GII.4 VP1 were pooled into two-dimensional matrices and used to identify putative T cell epitopes. Seven of the eight subjects produced IFN-γ in response to the peptides and five subjects produced IFN-γ in response to the VLPs of the same origin. In general, stronger T cell responses were induced with the peptides in each donor compared to the VLPs. A CD8+ T cell epitope in the shell domain of the VP1 (134SPSQVTMFPHIIVDVRQL151) was identified in two subjects, both having human leukocyte antigen (HLA)-A∗02:01 allele. To our knowledge, this is the first report using synthetic peptides to study NoV-specific T cell responses in human subjects and identify T cell epitopes.
Keywords: ELISPOT IFN-gamma; PBMC; T cell epitope; VLP; cellular immunity; norovirus; peptide pools.
Figures

Similar articles
-
Type-specific and cross-reactive antibodies and T cell responses in norovirus VLP immunized mice are targeted both to conserved and variable domains of capsid VP1 protein.Mol Immunol. 2016 Oct;78:27-37. doi: 10.1016/j.molimm.2016.08.009. Epub 2016 Aug 29. Mol Immunol. 2016. PMID: 27573255
-
Identification of a First Human Norovirus CD8+ T Cell Epitope Restricted to HLA-A*0201 Allele.Front Immunol. 2018 Nov 27;9:2782. doi: 10.3389/fimmu.2018.02782. eCollection 2018. Front Immunol. 2018. PMID: 30542352 Free PMC article.
-
Functionality and avidity of norovirus-specific antibodies and T cells induced by GII.4 virus-like particles alone or co-administered with different genotypes.Vaccine. 2018 Jan 25;36(4):484-490. doi: 10.1016/j.vaccine.2017.12.009. Epub 2017 Dec 13. Vaccine. 2018. PMID: 29246474
-
The Antigenic Topology of Norovirus as Defined by B and T Cell Epitope Mapping: Implications for Universal Vaccines and Therapeutics.Viruses. 2019 May 10;11(5):432. doi: 10.3390/v11050432. Viruses. 2019. PMID: 31083353 Free PMC article. Review.
-
An Overview of Peptides and Peptide Pools for Antigen-Specific Stimulation in T-Cell Assays.Methods Mol Biol. 2024;2768:29-50. doi: 10.1007/978-1-0716-3690-9_3. Methods Mol Biol. 2024. PMID: 38502386 Review.
Cited by
-
Rotavirus Inner Capsid VP6 Acts as an Adjuvant in Formulations with Particulate Antigens Only.Vaccines (Basel). 2020 Jul 7;8(3):365. doi: 10.3390/vaccines8030365. Vaccines (Basel). 2020. PMID: 32645976 Free PMC article.
-
Development of a broad-spectrum therapeutic Fc-nanobody for human noroviruses.J Virol. 2024 Jul 23;98(7):e0070724. doi: 10.1128/jvi.00707-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953655 Free PMC article.
-
A Soluble Version of Nipah Virus Glycoprotein G Delivered by Vaccinia Virus MVA Activates Specific CD8 and CD4 T Cells in Mice.Viruses. 2019 Dec 24;12(1):26. doi: 10.3390/v12010026. Viruses. 2019. PMID: 31878180 Free PMC article.
-
Advances in Human Norovirus Vaccine Research.Vaccines (Basel). 2021 Jul 2;9(7):732. doi: 10.3390/vaccines9070732. Vaccines (Basel). 2021. PMID: 34358148 Free PMC article. Review.
-
Human rhinovirus-specific CD8 T cell responses target conserved and unusual epitopes.FASEB J. 2021 Jan;35(1):e21208. doi: 10.1096/fj.202002165R. Epub 2020 Nov 23. FASEB J. 2021. PMID: 33230881 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

