Inward-rectifying K+ (Kir2) leak conductance dampens the excitability of lamina I projection neurons in the neonatal rat
- PMID: 27751963
- PMCID: PMC5118118
- DOI: 10.1016/j.neuroscience.2016.10.027
Inward-rectifying K+ (Kir2) leak conductance dampens the excitability of lamina I projection neurons in the neonatal rat
Abstract
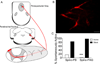
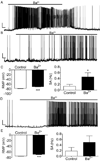
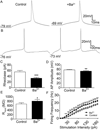
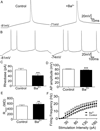
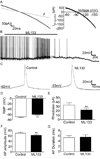
Spinal lamina I projection neurons serve as a major conduit by which noxious stimuli detected in the periphery are transmitted to nociceptive circuits in the brain, including the parabrachial nucleus (PB) and the periaqueductal gray (PAG). While neonatal spino-PB neurons are more than twice as likely to exhibit spontaneous activity compared to spino-PAG neurons, the underlying mechanisms remain unclear since nothing is known about the voltage-independent (i.e. 'leak') ion channels expressed by these distinct populations during early life. To begin identifying these key leak conductances, the present study investigated the role of classical inward-rectifying K+ (Kir2) channels in the regulation of intrinsic excitability in neonatal rat spino-PB and spino-PAG neurons. The data demonstrate that a reduction in Kir2-mediated conductance by external BaCl2 significantly enhanced intrinsic membrane excitability in both groups. Similar results were observed in spino-PB neurons following Kir2 channel block with the selective antagonist ML133. In addition, voltage-clamp experiments showed that spino-PB and spino-PAG neurons express similar amounts of Kir2 current during the early postnatal period, suggesting that the differences in the prevalence of spontaneous activity between the two populations are not explained by differential expression of Kir2 channels. Overall, the results indicate that Kir2-mediated conductance tonically dampens the firing of multiple subpopulations of lamina I projection neurons during early life. Therefore, Kir2 channels are positioned to tightly shape the output of the immature spinal nociceptive circuit and thus regulate the ascending flow of nociceptive information to the developing brain, which has important functional implications for pediatric pain.
Keywords: intrinsic excitability; leak conductance; projection neuron; superficial dorsal horn.
Copyright © 2016 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Intrinsic burst-firing in lamina I spinoparabrachial neurons during adolescence.Neurosci Lett. 2021 Apr 17;750:135794. doi: 10.1016/j.neulet.2021.135794. Epub 2021 Mar 2. Neurosci Lett. 2021. PMID: 33667599 Free PMC article.
-
Connectivity of pacemaker neurons in the neonatal rat superficial dorsal horn.J Comp Neurol. 2015 May 1;523(7):1038-1053. doi: 10.1002/cne.23706. Epub 2015 Feb 17. J Comp Neurol. 2015. PMID: 25380417 Free PMC article.
-
Developmental regulation of membrane excitability in rat spinal lamina I projection neurons.J Neurophysiol. 2012 May;107(10):2604-14. doi: 10.1152/jn.00899.2011. Epub 2012 Feb 15. J Neurophysiol. 2012. PMID: 22338021 Free PMC article.
-
Pacemaker Neurons and the Development of Nociception.Neuroscientist. 2014 Jun;20(3):197-202. doi: 10.1177/1073858414521499. Epub 2014 Feb 7. Neuroscientist. 2014. PMID: 24510073 Free PMC article. Review.
-
Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord.Brain Res Bull. 2000 Nov 15;53(5):649-59. doi: 10.1016/s0361-9230(00)00398-1. Brain Res Bull. 2000. PMID: 11165800 Review.
Cited by
-
Roles of K+ and cation channels in ORL-1 receptor-mediated depression of neuronal excitability and epileptic activities in the medial entorhinal cortex.Neuropharmacology. 2019 Jun;151:144-158. doi: 10.1016/j.neuropharm.2019.04.017. Epub 2019 Apr 15. Neuropharmacology. 2019. PMID: 30998945 Free PMC article.
-
Oxytocin receptors excite lateral nucleus of central amygdala by phospholipase Cβ- and protein kinase C-dependent depression of inwardly rectifying K+ channels.J Physiol. 2020 Aug;598(16):3501-3520. doi: 10.1113/JP279457. Epub 2020 Jun 14. J Physiol. 2020. PMID: 32458437 Free PMC article.
-
Intrinsic burst-firing in lamina I spinoparabrachial neurons during adolescence.Neurosci Lett. 2021 Apr 17;750:135794. doi: 10.1016/j.neulet.2021.135794. Epub 2021 Mar 2. Neurosci Lett. 2021. PMID: 33667599 Free PMC article.
-
Roles of PLCβ, PIP2 , and GIRK channels in arginine vasopressin-elicited excitation of CA1 pyramidal neurons.J Cell Physiol. 2022 Jan;237(1):660-674. doi: 10.1002/jcp.30535. Epub 2021 Jul 20. J Cell Physiol. 2022. PMID: 34287874 Free PMC article.
-
Activation of V1a vasopressin receptors excite subicular pyramidal neurons by activating TRPV1 and depressing GIRK channels.Neuropharmacology. 2021 Jun 1;190:108565. doi: 10.1016/j.neuropharm.2021.108565. Epub 2021 Apr 20. Neuropharmacology. 2021. PMID: 33891950 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

