Transgenic expression of cyclooxygenase-2 (COX2) causes premature aging phenotypes in mice
- PMID: 27750221
- PMCID: PMC5115895
- DOI: 10.18632/aging.101060
Transgenic expression of cyclooxygenase-2 (COX2) causes premature aging phenotypes in mice
Abstract
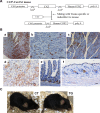
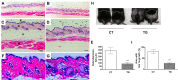
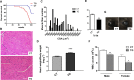
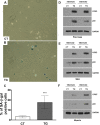
Cyclooxygenase (COX) is a key enzyme in the biosynthesis of prostanoids, lipid signaling molecules that regulate various physiological processes. COX2, one of the isoforms of COX, is highly inducible in response to a wide variety of cellular and environmental stresses. Increased COX2 expression is thought to play a role in the pathogenesis of many age-related diseases. COX2 expression is also reported to be increased in the tissues of aged humans and mice, which suggests the involvement of COX2 in the aging process. However, it is not clear whether the increased COX2 expression is causal to or a result of aging. We have now addressed this question by creating an inducible COX2 transgenic mouse model. Here we show that post-natal expression of COX2 led to a panel of aging-related phenotypes. The expression of p16, p53, and phospho-H2AX was increased in the tissues of COX2 transgenic mice. Additionally, adult mouse lung fibroblasts from COX2 transgenic mice exhibited increased expression of the senescence-associated β-galactosidase. Our study reveals that the increased COX2 expression has an impact on the aging process and suggests that modulation of COX2 and its downstream signaling may be an approach for intervention of age-related disorders.
Keywords: COX2; TP53; p16; premature aging; prostaglandin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The cyclooxygenase-2-prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts.Am J Respir Crit Care Med. 2013 Apr 1;187(7):703-14. doi: 10.1164/rccm.201208-1361OC. Am J Respir Crit Care Med. 2013. PMID: 23328527
-
The ATF6α arm of the Unfolded Protein Response mediates replicative senescence in human fibroblasts through a COX2/prostaglandin E2 intracrine pathway.Mech Ageing Dev. 2018 Mar;170:82-91. doi: 10.1016/j.mad.2017.08.003. Epub 2017 Aug 10. Mech Ageing Dev. 2018. PMID: 28803844
-
p63 deficiency activates a program of cellular senescence and leads to accelerated aging.Genes Dev. 2005 Sep 1;19(17):1986-99. doi: 10.1101/gad.342305. Epub 2005 Aug 17. Genes Dev. 2005. PMID: 16107615 Free PMC article.
-
[The role of lamins and mutations of LMNA gene in physiological and premature aging].Postepy Biochem. 2007;53(1):46-52. Postepy Biochem. 2007. PMID: 17718387 Review. Polish.
-
Cyclooxygenase 2: protein-protein interactions and posttranslational modifications.Physiol Genomics. 2017 Nov 1;49(11):667-681. doi: 10.1152/physiolgenomics.00086.2017. Epub 2017 Sep 22. Physiol Genomics. 2017. PMID: 28939645 Free PMC article. Review.
Cited by
-
Candesartan Neuroprotection in Rat Primary Neurons Negatively Correlates with Aging and Senescence: a Transcriptomic Analysis.Mol Neurobiol. 2020 Mar;57(3):1656-1673. doi: 10.1007/s12035-019-01800-9. Epub 2019 Dec 7. Mol Neurobiol. 2020. PMID: 31811565 Free PMC article.
-
Mesenchymal Stem Cells (MSCs) Coculture Protects [Ca2+]i Orchestrated Oxidant Mediated Damage in Differentiated Neurons In Vitro.Cells. 2018 Dec 6;7(12):250. doi: 10.3390/cells7120250. Cells. 2018. PMID: 30563298 Free PMC article.
-
Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad.Cells. 2021 Nov 10;10(11):3114. doi: 10.3390/cells10113114. Cells. 2021. PMID: 34831334 Free PMC article. Review.
-
Aging of lymphoid stromal architecture impacts immune responses.Semin Immunol. 2023 Nov;70:101817. doi: 10.1016/j.smim.2023.101817. Epub 2023 Aug 10. Semin Immunol. 2023. PMID: 37572552 Free PMC article. Review.
-
Aspirin ameliorates the long-term adverse effects of doxorubicin through suppression of cellular senescence.FASEB Bioadv. 2019 Sep 9;1(9):579-590. doi: 10.1096/fba.2019-00041. eCollection 2019 Sep. FASEB Bioadv. 2019. PMID: 32123852 Free PMC article.
References
-
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. - PubMed
-
- Yang CM, Lee IT, Lin CC, Yang YL, Luo SF, Kou YR, Hsiao LD. Cigarette smoke extract induces COX-2 expression via a PKCalpha/c-Src/EGFR, PDGFR/PI3K/Akt/NF-kappaB pathway and p300 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L892–902. doi: 10.1152/ajplung.00151.2009. - DOI - PubMed
-
- Park YK, Hong H, Jang BC. Transcriptional and translational regulation of COX-2 expression by cadmium in C6 glioma cells. Int J Mol Med. 2012;30:960–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

