Host-induced gene silencing compromises Verticillium wilt in tomato and Arabidopsis
- PMID: 27749994
- PMCID: PMC6638114
- DOI: 10.1111/mpp.12500
Host-induced gene silencing compromises Verticillium wilt in tomato and Arabidopsis
Abstract
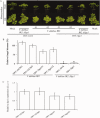
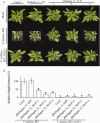
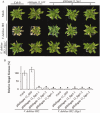
Verticillium wilt, caused by soil-borne fungi of the genus Verticillium, is an economically important disease that affects a wide range of host plants. Unfortunately, host resistance against Verticillium wilts is not available for many plant species, and the disease is notoriously difficult to combat. Host-induced gene silencing (HIGS) is an RNA interference (RNAi)-based process in which small RNAs are produced by the host plant to target parasite transcripts. HIGS has emerged as a promising strategy for the improvement of plant resistance against pathogens by silencing genes that are essential for these pathogens. Here, we assessed whether HIGS can be utilized to suppress Verticillium wilt disease by silencing three previously identified virulence genes of V. dahliae (encoding Ave1, Sge1 and NLP1) through the host plants tomato and Arabidopsis. In transient assays, tomato plants were agroinfiltrated with Tobacco rattle virus (TRV) constructs to target V. dahliae transcripts. Subsequent V. dahliae inoculation revealed the suppression of Verticillium wilt disease on treatment with only one of the three TRV constructs. Next, expression of RNAi constructs targeting transcripts of the same three V. dahliae virulence genes was pursued in stable transgenic Arabidopsis thaliana plants. In this host, V. dahliae inoculation revealed reduced Verticillium wilt disease in two of the three targets. Thus, our study suggests that, depending on the target gene chosen, HIGS against V. dahliae is operational in tomato and A. thaliana plants and may be exploited to engineer resistance in Verticillium wilt-susceptible crops.
Keywords: HIGS; RNAi; Verticillium; virulence gene.
© 2016 BSPP AND JOHN WILEY & SONS LTD.
Figures




Similar articles
-
Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis.Plant Physiol. 2011 Aug;156(4):2255-65. doi: 10.1104/pp.111.180067. Epub 2011 May 26. Plant Physiol. 2011. PMID: 21617027 Free PMC article.
-
Broad taxonomic characterization of Verticillium wilt resistance genes reveals an ancient origin of the tomato Ve1 immune receptor.Mol Plant Pathol. 2017 Feb;18(2):195-209. doi: 10.1111/mpp.12390. Epub 2016 Jun 9. Mol Plant Pathol. 2017. PMID: 26946045 Free PMC article.
-
Cotton polyamine oxidase is required for spermine and camalexin signalling in the defence response to Verticillium dahliae.Plant J. 2015 Sep;83(6):962-75. doi: 10.1111/tpj.12941. Epub 2015 Aug 26. Plant J. 2015. PMID: 26221980
-
Interactions between Verticillium dahliae and its host: vegetative growth, pathogenicity, plant immunity.Appl Microbiol Biotechnol. 2014 Aug;98(16):6921-32. doi: 10.1007/s00253-014-5863-8. Epub 2014 Jun 15. Appl Microbiol Biotechnol. 2014. PMID: 24928658 Review.
-
Insights to Gossypium defense response against Verticillium dahliae: the Cotton Cancer.Funct Integr Genomics. 2023 May 1;23(2):142. doi: 10.1007/s10142-023-01065-5. Funct Integr Genomics. 2023. PMID: 37121989 Review.
Cited by
-
Why Do We Need Alternative Methods for Fungal Disease Management in Plants?Plants (Basel). 2023 Nov 10;12(22):3822. doi: 10.3390/plants12223822. Plants (Basel). 2023. PMID: 38005718 Free PMC article. Review.
-
Small RNA-based plant protection against diseases.Front Plant Sci. 2022 Aug 18;13:951097. doi: 10.3389/fpls.2022.951097. eCollection 2022. Front Plant Sci. 2022. PMID: 36061762 Free PMC article. Review.
-
Roles of small RNAs in crop disease resistance.Stress Biol. 2021 Aug 18;1(1):6. doi: 10.1007/s44154-021-00005-2. Stress Biol. 2021. PMID: 37676520 Free PMC article. Review.
-
Threat at One End of the Plant: What Travels to Inform the Other Parts?Int J Mol Sci. 2021 Mar 19;22(6):3152. doi: 10.3390/ijms22063152. Int J Mol Sci. 2021. PMID: 33808792 Free PMC article. Review.
-
Host-Induced Gene Silencing of a Multifunction Gene Sscnd1 Enhances Plant Resistance Against Sclerotinia sclerotiorum.Front Microbiol. 2021 Oct 8;12:693334. doi: 10.3389/fmicb.2021.693334. eCollection 2021. Front Microbiol. 2021. PMID: 34690946 Free PMC article.
References
-
- Andrade, C. , Tinoco, M. , Rieth, A. , Maia, F. and Aragão, F. (2015) Host‐induced gene silencing in the necrotrophic fungal pathogen Sclerotinia sclerotiorum . Plant Pathol. 65, 626–632.
-
- Barrow, J.R. (1970) Heterozygosity in inheritance of Verticillium wilt tolerance in cotton. Phytopathology, 60, 301–303.
-
- Baulcombe, D. (2005) RNA silencing. Trends Biochem. Sci. 30, 290–293. - PubMed
-
- Baum, J.A. , Bogaert, T. , Clinton, W. , Heck, G.R. , Feldmann, P. , Ilagan, O. , Johnson, S. , Plaetinck, G. , Munyikwa, T. and Pleau, M. (2007) Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 25, 1322–1326. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

