Evaluating the effectiveness and safety of ursodeoxycholic acid in treatment of intrahepatic cholestasis of pregnancy: A meta-analysis (a prisma-compliant study)
- PMID: 27749550
- PMCID: PMC5059052
- DOI: 10.1097/MD.0000000000004949
Evaluating the effectiveness and safety of ursodeoxycholic acid in treatment of intrahepatic cholestasis of pregnancy: A meta-analysis (a prisma-compliant study)
Erratum in
-
Erratum: Evaluating the effectiveness and safety of ursodeoxycholic acid in treatment of intrahepatic cholestasis of pregnancy: A meta-analysis (a prisma-compliant study): Erratum.Medicine (Baltimore). 2017 Jan 20;96(3):e6031. doi: 10.1097/MD.0000000000006031. eCollection 2017 Jan. Medicine (Baltimore). 2017. PMID: 31305700 Free PMC article.
Abstract
Background: Intrahepatic cholestasis of pregnancy (ICP) is a specific pregnancy-related disorder without standard medical therapies. Ursodeoxycholic acid (UDCA) is the most used medicine, but the efficacy and safety of UDCA remain uncertain. Several meta-analyses had been made to assess the effects of UDCA in ICP. However, the samples were not large enough to convince obstetricians to use UDCA. We conducted a meta-analysis to evaluate the effects and safety of UDCA in patients with ICP, which included only randomized controlled trials (RCTs).
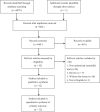
Methods: Six databases were searched. The search terms were "ursodeoxycholicacid," "therapy," "management," "treatment," "intrahepatic cholestasis of pregnancy," "obstetric cholestasis," "recurrent jaundice of pregnancy," "pruritus gravidarum," "idiopathic jaundice of pregnancy," "intrahepatic jaundice of pregnancy," and "icterus gravidarum."Randomized controlled trials of UDCA versus control groups (included using other medicines) among patients with ICP were included. The primary outcomes were improved pruritus scores and liver function. Secondary outcomes were the maternal and fetal outcomes in patients with ICP.Data were extracted from included RCTs. The Mantel-Haenzel random-effects model or fixed-effects model was used for meta-analysis.
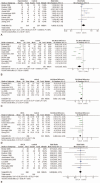
Results: A total of 12 RCTs involving 662 patients were included in the meta-analysis. In pooled analyses that compared UDCA with all controls, UDCA was associated with resolution of pruritus (risk ratio [RR], 1.68; 95% confidence interval [CI],1.12-2.52; P = 0.01),decrease of serum levels of alanine aminotransferase (ALT) (standardized mean difference (SMD), -1.36; 95% CI, -2.08 to -0.63; P <0.001), reduced serum levels of bile acid (SMD, -0.68; 95% CI, -1.15 to -0.20; P <0.001), fewer premature births (RR, 0.56; 95% CI, 0.43-0.72; P <0.001),reduced fetal distress (RR, 0.68; 95% CI, 0.49-0.94; P = 0.02), high Apgar scores at 5 minutes (RR, 0.44; 95% CI, 0.24-0.82; P = 0.009), less frequent respiratory distress syndrome (RDS) (RR, 0.33; 95% CI, 0.13-0.86; P = 0.02), and fewer neonates in the intensive care unit (NICU) (RR, 0.55; 95% CI, 0.35-0.87; P <0.05), increased gestational age (SMD,0.44; 95% CI, 0.26-0.63; P <0.001), and birth weight (SMD, 0.21; 95% CI, 0.02-0.40; P = 0.03). There were no differences in meconium staining and intrauterine growth retardation (IUGR) between the groups (P >0.05). No trials reported adverse effects on mothers and fetuses except nausea and emesis.
Conclusion: UDCA is effective and safe to improve pruritus and liver function in ICP. UDCA also reduced adverse maternal and fetal outcomes in pregnant women with ICP.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis.Gastroenterology. 2012 Dec;143(6):1492-501. doi: 10.1053/j.gastro.2012.08.004. Epub 2012 Aug 11. Gastroenterology. 2012. PMID: 22892336 Review.
-
Ursodeoxycholic acid versus placebo in the treatment of women with intrahepatic cholestasis of pregnancy (ICP) to improve perinatal outcomes: protocol for a randomised controlled trial (PITCHES).Trials. 2018 Nov 27;19(1):657. doi: 10.1186/s13063-018-3018-4. Trials. 2018. PMID: 30482254 Free PMC article.
-
Pregnancy course in patients with intrahepatic cholestasis of pregnancy treated with very low doses of ursodeoxycholic acid.Scand J Gastroenterol. 2016 Jan;51(1):78-85. doi: 10.3109/00365521.2015.1064990. Epub 2015 Jul 8. Scand J Gastroenterol. 2016. PMID: 26152830
-
Intrahepatic cholestasis of pregnancy: observational study of the treatment with low-dose ursodeoxycholic acid.BMC Gastroenterol. 2015 Jul 29;15:92. doi: 10.1186/s12876-015-0324-0. BMC Gastroenterol. 2015. PMID: 26215400 Free PMC article.
-
Interventions for treating cholestasis in pregnancy.Cochrane Database Syst Rev. 2013 Jun 24;2013(6):CD000493. doi: 10.1002/14651858.CD000493.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jul 27;7:CD000493. doi: 10.1002/14651858.CD000493.pub3 PMID: 23794285 Free PMC article. Updated. Review.
Cited by
-
Obeticholic acid improves fetal bile acid profile in a mouse model of gestational hypercholanemia.Am J Physiol Gastrointest Liver Physiol. 2020 Aug 1;319(2):G197-G211. doi: 10.1152/ajpgi.00126.2020. Epub 2020 Jun 29. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32597707 Free PMC article.
-
The prevalence and pregnancy outcomes of intrahepatic cholestasis of pregnancy: A retrospective clinical audit review.Obstet Med. 2019 Sep;12(3):123-128. doi: 10.1177/1753495X18797749. Epub 2018 Oct 25. Obstet Med. 2019. PMID: 31523268 Free PMC article.
-
Pharmacological interventions for treating intrahepatic cholestasis of pregnancy.Cochrane Database Syst Rev. 2020 Jul 27;7(7):CD000493. doi: 10.1002/14651858.CD000493.pub3. Cochrane Database Syst Rev. 2020. PMID: 32716060 Free PMC article.
-
Liver Diseases in the Parturient.Indian J Crit Care Med. 2021 Dec;25(Suppl 3):S248-S254. doi: 10.5005/jp-journals-10071-24027. Indian J Crit Care Med. 2021. PMID: 35615617 Free PMC article.
-
The biochemical diagnosis of intrahepatic cholestasis of pregnancy.Obstet Med. 2019 Jun;12(2):76-78. doi: 10.1177/1753495X18795979. Epub 2018 Nov 4. Obstet Med. 2019. PMID: 31217811 Free PMC article.
References
-
- Diken Z, Usta IM, Nassar AH. A clinical approach to intrahepatic cholestasis of pregnancy. Am J Perinatol 2014; 31:1–8. - PubMed
-
- Wei J, Wang H, Yang X, et al. Altered gene profile of placenta from women with intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet 2010; 281:801–810. - PubMed
-
- Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol 2014; 124:120–133. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

