Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition
- PMID: 27735939
- PMCID: PMC5133974
- DOI: 10.1038/cddis.2016.305
Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition
Erratum in
-
Correction to: Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition.Cell Death Dis. 2018 Sep 5;9(9):902. doi: 10.1038/s41419-018-0911-4. Cell Death Dis. 2018. PMID: 30185772 Free PMC article.
Retraction in
-
Retraction Note to: Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition.Cell Death Dis. 2021 Mar 15;12(3):281. doi: 10.1038/s41419-021-03529-7. Cell Death Dis. 2021. PMID: 33723213 Free PMC article. No abstract available.
Abstract
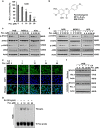
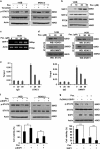
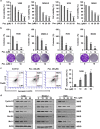
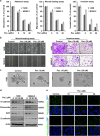
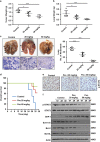
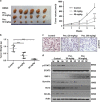
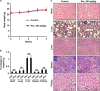
Signal transducer and activator of transcription 3 (STAT3) has important roles in cancer aggressiveness and has been confirmed as an attractive target for cancer therapy. In this study, we used a dual-luciferase assay to identify that pectolinarigenin inhibited STAT3 activity. Further studies showed pectolinarigenin inhibited constitutive and interleukin-6-induced STAT3 signaling, diminished the accumulation of STAT3 in the nucleus and blocked STAT3 DNA-binding activity in osteosarcoma cells. Mechanism investigations indicated that pectolinarigenin disturbed the STAT3/DNA methyltransferase 1/HDAC1 histone deacetylase 1 complex formation in the promoter region of SHP-1, which reversely mediates STAT3 signaling, leading to the upregulation of SHP-1 expression in osteosarcoma. We also found pectolinarigenin significantly suppressed osteosarcoma cell proliferation, induced apoptosis and reduced the level of STAT3 downstream proteins cyclin D1, Survivin, B-cell lymphoma 2 (Bcl-2), B-cell lymphoma extra-large (Bcl-xl) and myeloid cell leukemia 1 (Mcl-1). In addition, pectolinarigenin inhibited migration, invasion and reserved epithelial-mesenchymal transition (EMT) phenotype in osteosarcoma cells. In spontaneous and patient-derived xenograft models of osteosarcoma, we identified administration (intraperitoneal) of pectolinarigenin (20 mg/kg/2 days and 50 mg/kg/2 days) blocked STAT3 activation and impaired tumor growth and metastasis with superior pharmacodynamic properties. Taken together, our findings demonstrate that pectolinarigenin may be a candidate for osteosarcoma intervention linked to its STAT3 signaling inhibitory activity.
Figures
Similar articles
-
Pectolinarigenin acts as a potential anti-osteosarcoma agent via mediating SHP-1/JAK2/STAT3 signaling.Biomed Pharmacother. 2022 Sep;153:113323. doi: 10.1016/j.biopha.2022.113323. Epub 2022 Jun 22. Biomed Pharmacother. 2022. PMID: 35752008
-
Allicin Inhibits Proliferation and Invasion in Vitro and in Vivo via SHP-1-Mediated STAT3 Signaling in Cholangiocarcinoma.Cell Physiol Biochem. 2018;47(2):641-653. doi: 10.1159/000490019. Epub 2018 May 22. Cell Physiol Biochem. 2018. PMID: 29794468
-
Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases.Cancer Lett. 2014 Apr 1;345(1):140-8. doi: 10.1016/j.canlet.2013.12.008. Epub 2013 Dec 11. Cancer Lett. 2014. PMID: 24333736
-
Inhibiting STAT3 signaling pathway by natural products for cancer prevention and therapy: In vitro and in vivo activity and mechanisms of action.Pharmacol Res. 2022 Aug;182:106357. doi: 10.1016/j.phrs.2022.106357. Epub 2022 Jul 19. Pharmacol Res. 2022. PMID: 35868477 Review.
-
Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship?Ann N Y Acad Sci. 2009 Aug;1171:59-76. doi: 10.1111/j.1749-6632.2009.04911.x. Ann N Y Acad Sci. 2009. PMID: 19723038 Free PMC article. Review.
Cited by
-
Targeting the Wnt/β-catenin pathway in human osteosarcoma cells.Oncotarget. 2018 Dec 4;9(95):36780-36792. doi: 10.18632/oncotarget.26377. eCollection 2018 Dec 4. Oncotarget. 2018. PMID: 30613366 Free PMC article.
-
Rhein shows potent efficacy against non-small-cell lung cancer through inhibiting the STAT3 pathway.Cancer Manag Res. 2019 Feb 1;11:1167-1176. doi: 10.2147/CMAR.S171517. eCollection 2019. Cancer Manag Res. 2019. PMID: 30774444 Free PMC article.
-
Association of Diabetes Mellitus and Alcohol Abuse with Cancer: Molecular Mechanisms and Clinical Significance.Cells. 2021 Nov 8;10(11):3077. doi: 10.3390/cells10113077. Cells. 2021. PMID: 34831299 Free PMC article. Review.
-
Attenuation of STAT3 Phosphorylation Promotes Apoptosis and Chemosensitivity in Human Osteosarcoma Induced by Raddeanin A.Int J Biol Sci. 2019 Jan 24;15(3):668-679. doi: 10.7150/ijbs.30168. eCollection 2019. Int J Biol Sci. 2019. PMID: 30745853 Free PMC article.
-
Daurinol blocks breast and lung cancer metastasis and development by inhibition of focal adhesion kinase (FAK).Oncotarget. 2017 Jul 4;8(34):57058-57071. doi: 10.18632/oncotarget.18983. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915654 Free PMC article.
References
-
- Deng J, Grande F, Neamati N. Small molecule inhibitors of Stat3 signaling pathway. Curr Cancer Drug Targets 2007; 7: 91–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

