Circumventing furin enhances factor VIII biological activity and ameliorates bleeding phenotypes in hemophilia models
- PMID: 27734034
- PMCID: PMC5053153
- DOI: 10.1172/jci.insight.89371
Circumventing furin enhances factor VIII biological activity and ameliorates bleeding phenotypes in hemophilia models
Abstract
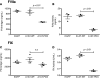
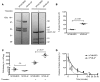
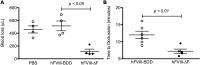
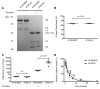
Processing by the proprotein convertase furin is believed to be critical for the biological activity of multiple proteins involved in hemostasis, including coagulation factor VIII (FVIII). This belief prompted the retention of the furin recognition motif (amino acids 1645-1648) in the design of B-domain-deleted FVIII (FVIII-BDD) products in current clinical use and in the drug development pipeline, as well as in experimental FVIII gene therapy strategies. Here, we report that processing by furin is in fact deleterious to FVIII-BDD secretion and procoagulant activity. Inhibition of furin increases the secretion and decreases the intracellular retention of FVIII-BDD protein in mammalian cells. Our new variant (FVIII-ΔF), in which this recognition motif is removed, efficiently circumvents furin. FVIII-ΔF demonstrates increased recombinant protein yields, enhanced clotting activity, and higher circulating FVIII levels after adeno-associated viral vector-based liver gene therapy in a murine model of severe hemophilia A (HA) compared with FVIII-BDD. Moreover, we observed an amelioration of the bleeding phenotype in severe HA dogs with sustained therapeutic FVIII levels after FVIII-ΔF gene therapy at a lower vector dose than previously employed in this model. The immunogenicity of FVIII-ΔF did not differ from that of FVIII-BDD as a protein or a gene therapeutic. Thus, contrary to previous suppositions, FVIII variants that can avoid furin processing are likely to have enhanced translational potential for HA therapy.
Figures





Similar articles
-
Novel factor VIII variants with a modified furin cleavage site improve the efficacy of gene therapy for hemophilia A.J Thromb Haemost. 2017 Jan;15(1):110-121. doi: 10.1111/jth.13543. Epub 2016 Nov 25. J Thromb Haemost. 2017. PMID: 27749002 Free PMC article.
-
Minimal modification in the factor VIII B-domain sequence ameliorates the murine hemophilia A phenotype.Blood. 2013 May 23;121(21):4396-403. doi: 10.1182/blood-2012-10-464164. Epub 2013 Jan 31. Blood. 2013. PMID: 23372167 Free PMC article.
-
Phenotype correction of hemophilia A mice with adeno-associated virus vectors carrying the B domain-deleted canine factor VIII gene.Thromb Res. 2006;118(5):627-35. doi: 10.1016/j.thromres.2005.11.006. Epub 2005 Dec 20. Thromb Res. 2006. PMID: 16371232
-
Hemophilia Gene Therapy: Ready for Prime Time?Hum Gene Ther. 2017 Nov;28(11):1013-1023. doi: 10.1089/hum.2017.116. Epub 2017 Aug 3. Hum Gene Ther. 2017. PMID: 28793786 Review.
-
Translational Potential of Immune Tolerance Induction by AAV Liver-Directed Factor VIII Gene Therapy for Hemophilia A.Front Immunol. 2020 Apr 28;11:618. doi: 10.3389/fimmu.2020.00618. eCollection 2020. Front Immunol. 2020. PMID: 32425925 Free PMC article. Review.
Cited by
-
Update on clinical gene therapy for hemophilia.Blood. 2019 Jan 31;133(5):407-414. doi: 10.1182/blood-2018-07-820720. Epub 2018 Dec 17. Blood. 2019. PMID: 30559260 Free PMC article. Review.
-
Analysis of vector genome integrations in multicentric lymphoma after AAV gene therapy in a severe hemophilia A dog.Mol Ther Methods Clin Dev. 2023 Nov 14;31:101159. doi: 10.1016/j.omtm.2023.101159. eCollection 2023 Dec 14. Mol Ther Methods Clin Dev. 2023. PMID: 38094200 Free PMC article.
-
Functional Roles of Furin in Cardio-Cerebrovascular Diseases.ACS Pharmacol Transl Sci. 2024 Feb 7;7(3):570-585. doi: 10.1021/acsptsci.3c00325. eCollection 2024 Mar 8. ACS Pharmacol Transl Sci. 2024. PMID: 38481703 Free PMC article. Review.
-
Novel alternate hemostatic agents for patients with inhibitors: beyond bypass therapy.Hematology Am Soc Hematol Educ Program. 2017 Dec 8;2017(1):605-609. doi: 10.1182/asheducation-2017.1.605. Hematology Am Soc Hematol Educ Program. 2017. PMID: 29222310 Free PMC article. Review.
-
Correction of bleeding in experimental severe hemophilia A by systemic delivery of factor VIII-encoding mRNA.Haematologica. 2020 Apr;105(4):1129-1137. doi: 10.3324/haematol.2018.210583. Epub 2019 Jul 9. Haematologica. 2020. PMID: 31289204 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

