Tomosyn Negatively Regulates Arginine Vasopressin Secretion in Embryonic Stem Cell-Derived Neurons
- PMID: 27732637
- PMCID: PMC5061411
- DOI: 10.1371/journal.pone.0164544
Tomosyn Negatively Regulates Arginine Vasopressin Secretion in Embryonic Stem Cell-Derived Neurons
Abstract
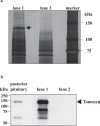
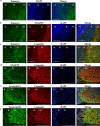
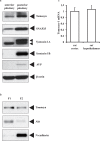
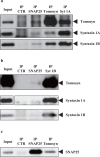
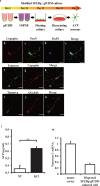
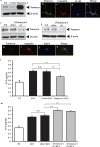
Arginine vasopressin (AVP) is secreted via exocytosis; however, the precise molecular mechanism underlying the exocytosis of AVP remains to be elucidated. To better understand the mechanisms of AVP secretion, in our study we have identified proteins that bind with a 25 kDa synaptosomal-associated protein (SNAP25). SNAP25 plays a crucial role in exocytosis, in the posterior pituitary. Embryonic stem (ES) cell-derived AVP neurons were established to investigate the functions of the identified proteins. Using glutathione S-transferase (GST)-pulldown assays and proteomic analyses, we identified tomosyn-1 (syntaxin-binding protein 5) as a SNAP25-binding protein in the posterior pituitary. Coimmunoprecipitation assays indicated that tomosyn formed N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes with SNAP25 and syntaxin1. Immunohistochemistry showed that tomosyn localized to the posterior pituitary. Mouse ES cells self-differentiated into AVP neurons (mES-AVP) that expressed tomosyn and two transmembrane SNARE proteins, including SNAP25 and syntaxin1. KCl increased AVP secretion in mES-AVP, and overexpression of tomosyn-1 reduced KCl-stimulated AVP secretion. Downregulation of tomosyn-1 with siRNA increased KCl-stimulated AVP secretion. These results suggested that tomosyn-1 negatively regulated AVP secretion in mES-AVP and further suggest the possibility of using mES-AVP culture systems to evaluate the role of synaptic proteins from AVP neurons.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Differential interaction of tomosyn with syntaxin and SNAP25 depends on domains in the WD40 β-propeller core and determines its inhibitory activity.J Biol Chem. 2014 Jun 13;289(24):17087-99. doi: 10.1074/jbc.M113.515296. Epub 2014 Apr 29. J Biol Chem. 2014. PMID: 24782308 Free PMC article.
-
The N- and C-terminal domains of tomosyn play distinct roles in soluble N-ethylmaleimide-sensitive factor attachment protein receptor binding and fusion regulation.J Biol Chem. 2014 Sep 12;289(37):25571-80. doi: 10.1074/jbc.M114.591487. Epub 2014 Jul 25. J Biol Chem. 2014. PMID: 25063806 Free PMC article.
-
Tomosyn-1 is involved in a post-docking event required for pancreatic beta-cell exocytosis.J Cell Sci. 2006 Jul 15;119(Pt 14):2912-20. doi: 10.1242/jcs.03037. Epub 2006 Jun 20. J Cell Sci. 2006. PMID: 16787939
-
SNAREs in neurons--beyond synaptic vesicle exocytosis (Review).Mol Membr Biol. 2006 Sep-Oct;23(5):377-84. doi: 10.1080/09687860600776734. Mol Membr Biol. 2006. PMID: 17060155 Review.
-
[Roles of tomosyn in regulated synaptic vesicle fusion].Tanpakushitsu Kakusan Koso. 2009 Sep;54(12 Suppl):1647-53. Tanpakushitsu Kakusan Koso. 2009. PMID: 21089602 Review. Japanese. No abstract available.
References
-
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81(3):1197–267. - PubMed
-
- Gainer H, Chin H. Molecular diversity in neurosecretion: reflections on the hypothalamo-neurohypophysial system. Cell Mol Neurobiol. 1998;18(2):211–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

