Studying Dynamic Myofiber Aggregate Reorientation in Dilated Cardiomyopathy Using In Vivo Magnetic Resonance Diffusion Tensor Imaging
- PMID: 27729361
- PMCID: PMC5068188
- DOI: 10.1161/CIRCIMAGING.116.005018
Studying Dynamic Myofiber Aggregate Reorientation in Dilated Cardiomyopathy Using In Vivo Magnetic Resonance Diffusion Tensor Imaging
Abstract
Background: The objective of this study is to assess the dynamic alterations of myocardial microstructure and strain between diastole and systole in patients with dilated cardiomyopathy relative to healthy controls using the magnetic resonance diffusion tensor imaging, myocardial tagging, and biomechanical modeling.
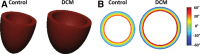
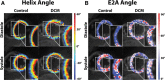
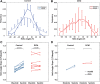
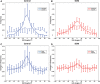
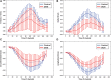
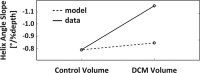
Methods and results: Dual heart-phase diffusion tensor imaging was successfully performed in 9 patients and 9 controls. Tagging data were acquired for the diffusion tensor strain correction and cardiac motion analysis. Mean diffusivity, fractional anisotropy, and myocyte aggregate orientations were compared between both cohorts. Cardiac function was assessed by left ventricular ejection fraction, torsion, and strain. Computational modeling was used to study the impact of cardiac shape on fiber reorientation and how fiber orientations affect strain. In patients with dilated cardiomyopathy, a more longitudinal orientation of diastolic myofiber aggregates was measured compared with controls. Although a significant steepening of helix angles (HAs) during contraction was found in the controls, consistent change in HAs during contraction was absent in patients. Left ventricular ejection fraction, cardiac torsion, and strain were significantly lower in the patients compared with controls. Computational modeling revealed that the dilated heart results in reduced HA changes compared with a normal heart. Reduced torsion was found to be exacerbated by steeper HAs.
Conclusions: Diffusion tensor imaging revealed reduced reorientation of myofiber aggregates during cardiac contraction in patients with dilated cardiomyopathy relative to controls. Left ventricular remodeling seems to be an important factor in the changes to myocyte orientation. Steeper HAs are coupled with a worsening in strain and torsion. Overall, the findings provide new insights into the structural alterations in patients with dilated cardiomyopathy.
Keywords: diffusion tensor imaging; dilated cardiomyopathy; magnetic resonance imaging; myocardium; myofiber architecture.
© 2016 The Authors.
Figures

Comment in
-
Magnetic Resonance Diffusion Tensor Imaging Provides New Insights Into the Microstructural Alterations in Dilated Cardiomyopathy.Circ Cardiovasc Imaging. 2016 Oct;9(10):e005593. doi: 10.1161/CIRCIMAGING.116.005593. Circ Cardiovasc Imaging. 2016. PMID: 27729369 Free PMC article. No abstract available.
Similar articles
-
Myocardial mesostructure and mesofunction.Am J Physiol Heart Circ Physiol. 2022 Aug 1;323(2):H257-H275. doi: 10.1152/ajpheart.00059.2022. Epub 2022 Jun 3. Am J Physiol Heart Circ Physiol. 2022. PMID: 35657613 Free PMC article. Review.
-
In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy.J Cardiovasc Magn Reson. 2014 Nov 12;16(1):87. doi: 10.1186/s12968-014-0087-8. J Cardiovasc Magn Reson. 2014. PMID: 25388867 Free PMC article.
-
Dual-phase cardiac diffusion tensor imaging with strain correction.PLoS One. 2014 Sep 5;9(9):e107159. doi: 10.1371/journal.pone.0107159. eCollection 2014. PLoS One. 2014. PMID: 25191900 Free PMC article.
-
Characterizing cardiac involvement in amyloidosis using cardiovascular magnetic resonance diffusion tensor imaging.J Cardiovasc Magn Reson. 2019 Sep 5;21(1):56. doi: 10.1186/s12968-019-0563-2. J Cardiovasc Magn Reson. 2019. PMID: 31484544 Free PMC article.
-
Diffusion Tensor Cardiovascular Magnetic Resonance Imaging: A Clinical Perspective.JACC Cardiovasc Imaging. 2020 May;13(5):1235-1255. doi: 10.1016/j.jcmg.2019.07.016. Epub 2019 Oct 11. JACC Cardiovasc Imaging. 2020. PMID: 31607663 Review.
Cited by
-
Impact of intraventricular septal fiber orientation on cardiac electromechanical function.Am J Physiol Heart Circ Physiol. 2022 Jun 1;322(6):H936-H952. doi: 10.1152/ajpheart.00050.2022. Epub 2022 Mar 18. Am J Physiol Heart Circ Physiol. 2022. PMID: 35302879 Free PMC article.
-
Diffusion tensor cardiovascular magnetic resonance in hypertrophic cardiomyopathy: a comparison of motion-compensated spin echo and stimulated echo techniques.MAGMA. 2020 Jun;33(3):331-342. doi: 10.1007/s10334-019-00799-3. Epub 2019 Nov 22. MAGMA. 2020. PMID: 31758419 Free PMC article.
-
Clinical Translation of Three-Dimensional Scar, Diffusion Tensor Imaging, Four-Dimensional Flow, and Quantitative Perfusion in Cardiac MRI: A Comprehensive Review.Front Cardiovasc Med. 2021 Jul 7;8:682027. doi: 10.3389/fcvm.2021.682027. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34307496 Free PMC article. Review.
-
Myocardial mesostructure and mesofunction.Am J Physiol Heart Circ Physiol. 2022 Aug 1;323(2):H257-H275. doi: 10.1152/ajpheart.00059.2022. Epub 2022 Jun 3. Am J Physiol Heart Circ Physiol. 2022. PMID: 35657613 Free PMC article. Review.
-
Myocardial Scar Delineation Using Diffusion Tensor Magnetic Resonance Tractography.J Am Heart Assoc. 2018 Feb 2;7(3):e007834. doi: 10.1161/JAHA.117.007834. J Am Heart Assoc. 2018. PMID: 29420216 Free PMC article.
References
-
- Kasper EK, Agema WR, Hutchins GM, Deckers JW, Hare JM, Baughman KL. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J Am Coll Cardiol. 1994;23:586–590. doi: 10.1016/0735-1097(94)90740-4. - PubMed
-
- Unverferth DV, Fetters JK, Unverferth BJ, Leier CV, Magorien RD, Arn AR, Baker PB. Human myocardial histologic characteristics in congestive heart failure. Circulation. 1983;68:1194–1200. doi: 10.1161/01.CIR.68.6.1194. - PubMed
-
- Wu A, Das S. Sudden death in idiopathic dilated cardiomyopathy. Am Hear J. 1992;124:1035–1045. - PubMed
-
- Gerdes AM, Capasso JM. Structural remodeling and mechanical dysfunction of cardiac myocytes in heart failure. J Mol Cell Cardiol. 1995;27:849–856. doi: 10.1016/0022-2828(95)90000-4. - PubMed
-
- Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–254. doi: 10.1016/0735-1097(92)90167-L. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

