Clinically Relevant Reactivation of Polyomavirus BK (BKPyV) in HLA-A02-Positive Renal Transplant Recipients Is Associated with Impaired Effector-Memory Differentiation of BKPyV-Specific CD8+ T Cells
- PMID: 27723787
- PMCID: PMC5056763
- DOI: 10.1371/journal.ppat.1005903
Clinically Relevant Reactivation of Polyomavirus BK (BKPyV) in HLA-A02-Positive Renal Transplant Recipients Is Associated with Impaired Effector-Memory Differentiation of BKPyV-Specific CD8+ T Cells
Abstract
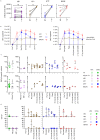
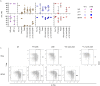
Polyomavirus BK (BKPyV) frequently reactivates in immunosuppressed renal transplant recipients (RTRs) and may lead to graft loss due to BKPyV-induced interstitial nephritis (BKVN). Little is known on the differentiation of CD8+ T cells targeting BKPyV in RTRs. Here we investigated whether BKPyV-specific CD8+ T cell differentiation differs in RTRs with varying degrees of BKPyV reactivation and/or BKVN. Using combinatorial encoding with tetramers carrying BKPyV major capsid protein (VP1) and large T antigen protein (LTAG) epitopes, we investigated CD8+ T cell responses to BKPyV in longitudinally obtained PBMC samples from 46 HLA-A02-positive RTRs and 20 healthy adults. We were also able to isolate BKPyV-specific CD8+ T cells from five renal allografts, two of which were affected by BKVN. Before transplantation, BKPyV-specific CD8+ T cells targeting VP1 and LTAG epitopes appeared predominantly as central-memory and CD27+/CD28+ effector-memory (TEM), and naïve-like PD-1-expressing cells, respectively. After viral reactivation, BKPyV-specific CD8+ T cells assumed CD28- TEM and TEMRA states in patients who were able to control BKPyV, whereas differentiation lagged behind in patients with severe viral reactivation or BKVN. Furthermore, VP1-specific CD69+/CD103+ tissue-resident memory (TRM) cells accumulated in BKVN-affected allografts but lacked signs of effector differentiation. In contrast, granzyme B-expressing effector cells were detected in allografts not affected by BKVN. In conclusion, effector-memory differentiation of BKPyV-specific CD8+ T cells in patients with high viral load or BKVN is impaired. Further characterization of the specific mechanisms behind this altered cellular differentiation is necessary to develop therapies that can prevent the emergence of BKVN.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Polyomavirus BK-Specific CD4+ T Cells Response to VP1 Stimulation in Kidney Transplant Recipients.Iran J Allergy Asthma Immunol. 2024 May 27;23(3):272-287. doi: 10.18502/ijaai.v23i3.15637. Iran J Allergy Asthma Immunol. 2024. PMID: 39422387
-
Pre-transplant immune factors may be associated with BK polyomavirus reactivation in kidney transplant recipients.PLoS One. 2017 May 31;12(5):e0177339. doi: 10.1371/journal.pone.0177339. eCollection 2017. PLoS One. 2017. PMID: 28562595 Free PMC article.
-
Functional characterization of BK virus-specific CD4+ T cells with cytotoxic potential in seropositive adults.Viral Immunol. 2007 Sep;20(3):379-88. doi: 10.1089/vim.2007.0030. Viral Immunol. 2007. PMID: 17931108
-
The effect of BK polyomavirus large T antigen on CD4 and CD8 T cells in kidney transplant recipients.Transpl Immunol. 2022 Oct;74:101655. doi: 10.1016/j.trim.2022.101655. Epub 2022 Jun 28. Transpl Immunol. 2022. PMID: 35777612 Review.
-
BK virus nephropathy in renal transplant recipients.Nephrology (Carlton). 2016 Aug;21(8):647-54. doi: 10.1111/nep.12728. Nephrology (Carlton). 2016. PMID: 26780694 Review.
Cited by
-
Tissue-resident memory T cells in the urogenital tract.Nat Rev Nephrol. 2022 Apr;18(4):209-223. doi: 10.1038/s41581-021-00525-0. Epub 2022 Jan 25. Nat Rev Nephrol. 2022. PMID: 35079143 Review.
-
Tissue-Specific Control of Tissue-Resident Memory T Cells.Crit Rev Immunol. 2018;38(2):79-103. doi: 10.1615/CritRevImmunol.2018025653. Crit Rev Immunol. 2018. PMID: 29953389 Free PMC article. Review.
-
CD8+ Resident Memory T Cells and Viral Infection.Front Immunol. 2018 Sep 19;9:2093. doi: 10.3389/fimmu.2018.02093. eCollection 2018. Front Immunol. 2018. PMID: 30283442 Free PMC article. Review.
-
Tissue-resident memory T cells in the kidney.Semin Immunopathol. 2022 Nov;44(6):801-811. doi: 10.1007/s00281-022-00927-7. Epub 2022 Apr 11. Semin Immunopathol. 2022. PMID: 35411437 Free PMC article. Review.
-
Early postoperative urinary MCP-1 as a potential biomarker predicting acute rejection in living donor kidney transplantation: a prospective cohort study.Sci Rep. 2021 Sep 22;11(1):18832. doi: 10.1038/s41598-021-98135-0. Sci Rep. 2021. PMID: 34552150 Free PMC article.
References
-
- van Aalderen MC, Heutinck KM, Huisman C, Ten Berge IJ. BK virus infection in transplant recipients: Clinical manifestations, treatment options and the immune response. NethJMed. 2012;70(4):172–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

