Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches
- PMID: 27721455
- PMCID: PMC5301156
- DOI: 10.1038/cmi.2016.39
Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches
Abstract
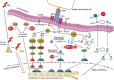
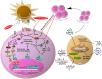
Almost every experimental treatment strategy using non-autologous cell, tissue or organ transplantation is tested in small and large animal models before clinical translation. Because these strategies require immunosuppression in most cases, immunosuppressive protocols are a key element in transplantation experiments. However, standard immunosuppressive protocols are often applied without detailed knowledge regarding their efficacy within the particular experimental setting and in the chosen model species. Optimization of such protocols is pertinent to the translation of experimental results to human patients and thus warrants further investigation. This review summarizes current knowledge regarding immunosuppressive drug classes as well as their dosages and application regimens with consideration of species-specific drug metabolization and side effects. It also summarizes contemporary knowledge of novel immunomodulatory strategies, such as the use of mesenchymal stem cells or antibodies. Thus, this review is intended to serve as a state-of-the-art compendium for researchers to refine applied experimental immunosuppression and immunomodulation strategies to enhance the predictive value of preclinical transplantation studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Drug delivery strategies for local immunomodulation in transplantation: Bridging the translational gap.Adv Drug Deliv Rev. 2024 Oct;213:115429. doi: 10.1016/j.addr.2024.115429. Epub 2024 Aug 13. Adv Drug Deliv Rev. 2024. PMID: 39142608 Review.
-
Evolutionary experience with immunosuppression in pediatric intestinal transplantation.J Pediatr Surg. 2005 Jan;40(1):274-9; discussion 279-80. doi: 10.1016/j.jpedsurg.2004.09.020. J Pediatr Surg. 2005. PMID: 15868597
-
Novel immunological strategies for islet transplantation.Pharmacol Res. 2015 Aug;98:69-75. doi: 10.1016/j.phrs.2014.06.016. Epub 2014 Jul 8. Pharmacol Res. 2015. PMID: 25014184 Review.
-
Immunomodulatory effects of stem cells.Periodontol 2000. 2013 Oct;63(1):198-216. doi: 10.1111/prd.12024. Periodontol 2000. 2013. PMID: 23931061 Review.
-
Crossing the bridge: large animal models in translational transplantation research.Immunol Rev. 2003 Dec;196:176-96. doi: 10.1046/j.1600-065x.2003.00081.x. Immunol Rev. 2003. PMID: 14617205 Review.
Cited by
-
Role of Regulatory T Cells in Intracerebral Hemorrhage.Mol Neurobiol. 2025 Jan;62(1):518-532. doi: 10.1007/s12035-024-04281-7. Epub 2024 Jun 14. Mol Neurobiol. 2025. PMID: 38877366 Review.
-
Using an Aluminum Hydroxide-Chitosan Matrix Increased the Vaccine Potential and Immune Response of Mice against Multi-Drug-Resistant Acinetobacter baumannii.Vaccines (Basel). 2023 Mar 16;11(3):669. doi: 10.3390/vaccines11030669. Vaccines (Basel). 2023. PMID: 36992253 Free PMC article.
-
Olfactory Mucosa Mesenchymal Stem Cells Alleviate Cerebral Ischemia/Reperfusion Injury Via Golgi Apparatus Secretory Pathway Ca2+ -ATPase Isoform1.Front Cell Dev Biol. 2020 Oct 30;8:586541. doi: 10.3389/fcell.2020.586541. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195239 Free PMC article.
-
Magnetic resonance imaging of human neural stem cells in rodent and primate brain.Stem Cells Transl Med. 2021 Jan;10(1):83-97. doi: 10.1002/sctm.20-0126. Epub 2020 Aug 25. Stem Cells Transl Med. 2021. PMID: 32841522 Free PMC article.
-
Apelin modulates inflammation and leukocyte recruitment in experimental autoimmune encephalomyelitis.Nat Commun. 2024 Jul 25;15(1):6282. doi: 10.1038/s41467-024-50540-5. Nat Commun. 2024. PMID: 39060233 Free PMC article.
References
-
- Kahan BD. Cyclosporine. N Engl J Med 1989; 321: 1725–1738. - PubMed
-
- Steffan J, Favrot C, Mueller R. A systematic review and meta-analysis of the efficacy and safety of cyclosporin for the treatment of atopic dermatitis in dogs. Vet Dermatol 2006; 17: 3–16. - PubMed
-
- Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev 2003; 196: 176–196. - PubMed
-
- Ptachcinski RJ, Burckart GJ, Venkataramanan R. Cyclosporine concentration determinations for monitoring and pharmacokinetic studies. J Clin Pharmacol 1986; 26: 358–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

